Multifunctional magneto-electric and exosome-loaded hydrogel enhances neuronal differentiation and immunoregulation through remote non-invasive electrical stimulation for neurological recovery after spinal cord injury
- PMID: 40104021
- PMCID: PMC11919302
- DOI: 10.1016/j.bioactmat.2025.02.034
Multifunctional magneto-electric and exosome-loaded hydrogel enhances neuronal differentiation and immunoregulation through remote non-invasive electrical stimulation for neurological recovery after spinal cord injury
Abstract
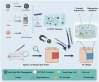
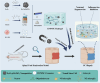
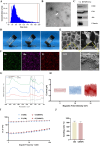
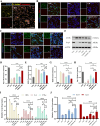
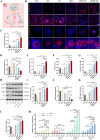
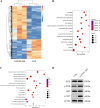
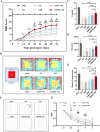
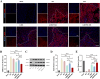
Intervention in the differentiation of neural stem cells (NSCs) is emerging as a highly promising approach for the treatment of spinal cord injury (SCI). However, NSCs at the injury site often suffer from low survival and uncontrolled differentiation. Whereas electrical stimulation has proven effective in regulating the fate of NSCs and promoting tissue repair, however, conventional electrical stimulation therapy has failed to be widely applied due to challenges such as invasiveness and technical complexity. To overcome these limitations, we developed a biomimetic magneto-electric hydrogel incorporating Fe3O4@BaTiO3 core-shell nanoparticles and human umbilical mesenchymal stem cell exosomes (HUMSC-Exos) around the concept of constructing remote noninvasive electrical stimulation for the synergistic treatment of SCI. The Fe3O4@BaTiO3 is activated by the peripheral magnetic field to generate electrical stimulation, which, in conjunction with the synergistic effects of HUMSC-Exos, significantly alleviates the early inflammatory response associated with SCI and enhances the regeneration of newborn neurons and axons, thereby creating favorable conditions for functional recovery post-SCI. Our findings indicate that applying this magneto-exosome hydrogel in a rat model of SCI leads to substantial functional recovery. This innovative combination represents a promising therapeutic strategy for SCI repair.
Keywords: HUMSC-Exos; Magneto-electric nanoparticles; Neuronal differentiation; Spinal cord injury repair; Tissue engineering.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
An injectable, self-healing, electroconductive hydrogel loaded with neural stem cells and donepezil for enhancing local therapy effect of spinal cord injury.J Biol Eng. 2023 Jul 24;17(1):48. doi: 10.1186/s13036-023-00368-2. J Biol Eng. 2023. PMID: 37488558 Free PMC article.
-
Synergistic effects of human umbilical cord mesenchymal stem cells/neural stem cells and epidural electrical stimulation on spinal cord injury rehabilitation.Sci Rep. 2024 Oct 30;14(1):26090. doi: 10.1038/s41598-024-75754-x. Sci Rep. 2024. PMID: 39478010 Free PMC article.
-
Coaxial 3D printing of hierarchical structured hydrogel scaffolds for on-demand repair of spinal cord injury.Acta Biomater. 2023 Sep 15;168:400-415. doi: 10.1016/j.actbio.2023.07.020. Epub 2023 Jul 20. Acta Biomater. 2023. PMID: 37479156
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Direct neuronal differentiation of neural stem cells for spinal cord injury repair.Stem Cells. 2021 Aug;39(8):1025-1032. doi: 10.1002/stem.3366. Epub 2021 Mar 5. Stem Cells. 2021. PMID: 33657255 Review.
Cited by
-
Exo-hydrogel therapy: a revolutionary approach to managing diabetic complications.J Nanobiotechnology. 2025 Aug 11;23(1):558. doi: 10.1186/s12951-025-03621-6. J Nanobiotechnology. 2025. PMID: 40790200 Free PMC article. Review.
-
Exosomes: a promising microenvironment modulator for spinal cord injury treatment.Int J Biol Sci. 2025 Jun 5;21(8):3791-3824. doi: 10.7150/ijbs.115242. eCollection 2025. Int J Biol Sci. 2025. PMID: 40520019 Free PMC article. Review.
References
-
- Karsy M., Hawryluk G. Modern medical management of spinal cord injury. Curr. Neurol. Neurosci. Rep. 2019;19:65. - PubMed
-
- Quadri S.A., Farooqui M., Ikram A., Zafar A., Khan M.A., Suriya S.S., Claus C.F., Fiani B., Rahman M., Ramachandran A., Armstrong I.I.T., Taqi M.A., Mortazavi M.M. Recent update on basic mechanisms of spinal cord injury. Neurosurg. Rev. 2020;43:425–441. - PubMed
-
- Hutson T.H., Di Giovanni S. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019;15:732–745. - PubMed
-
- Li X., Peng Z., Long L., Tuo Y., Wang L., Zhao X., Le W., Wan Y. Wnt4-modified NSC transplantation promotes functional recovery after spinal cord injury. FASEB J. 2020;34:82–94. - PubMed
LinkOut - more resources
Full Text Sources

