Inhibition of the NLRP3 inflammasome using MCC950 reduces vincristine-induced adverse effects in an acute lymphoblastic leukemia patient-derived xenograft model
- PMID: 40104043
- PMCID: PMC11915122
- DOI: 10.1002/hem3.70092
Inhibition of the NLRP3 inflammasome using MCC950 reduces vincristine-induced adverse effects in an acute lymphoblastic leukemia patient-derived xenograft model
Abstract
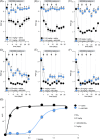
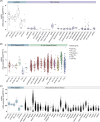
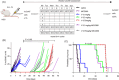
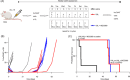
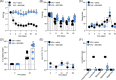
Vincristine is one of the most important chemotherapeutic drugs used to treat acute lymphoblastic leukemia (ALL). Unfortunately, vincristine often causes severe adverse effects, including sensory-motor neuropathies, weight loss, and overall decreased well-being, that are difficult to control and that decrease the quality of life and survival of patients. Recent studies demonstrate that sensory-motor adverse effects of vincristine are driven by neuroinflammatory processes, including the activation of the Nod-like receptor 3 (NLRP3) inflammasome. In this study, we aimed to test the effects of MCC950, a specific NLRP3 inhibitor, on the prevention of vincristine-induced adverse effects as well as tumor progression and vincristine efficacy in NOD/SCID/interleukin-2 receptor γ-negative mice patient-derived xenografts of ALL. We demonstrate that co-administration of MCC950 effectively prevented the development of mechanical allodynia, motor impairment, and weight loss and significantly improved the overall well-being of the animals without negatively impacting the in vivo efficacy of vincristine as a single agent or in combination with standard-of-care drugs. These results provide proof of principle that the adverse effects of vincristine chemotherapy can be prevented using NLRP3 inflammasome inhibitors and provide new options for the development of effective treatment strategies.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy‐induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020;38(28):3325‐3348. - PubMed
LinkOut - more resources
Full Text Sources
