Astrocyte-induced firing in primary afferent axons
- PMID: 40104051
- PMCID: PMC11914515
- DOI: 10.1016/j.isci.2025.112006
Astrocyte-induced firing in primary afferent axons
Abstract
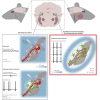
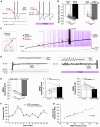
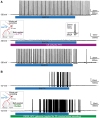
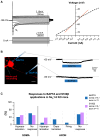
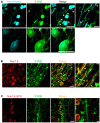
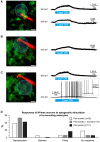
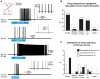
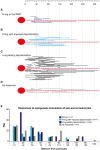
The large-caliber primary afferents innervating the spindles of the jaw-closing muscles have their cell bodies located centrally in the mesencephalic trigeminal nucleus (NVmes). We have shown, in an acid-induced jaw muscle chronic myalgia model, that these afferents exhibit increased excitability and ectopic discharges that emerge from subthreshold membrane oscillations (SMOs) supported by a persistent sodium current (I NaP) exquisitely sensitive to extracellular Ca2+-decreases. Here, we explore if the Ca2+-binding astrocytic protein, S100β, contributes to this hyperexcitability emergence and aim to localize the site where ectopic discharge arises using whole-cell patch-clamp recordings on mice brain slices. We found that astrocytes, by lowering [Ca2+]e at focal points along the axons of NVmes neurons through S100β, enhance the amplitude of the NaV1.6-dependent SMOs, leading to ectopic firing. These findings suggest a crucial role for astrocytes in excitability regulation and raise questions about this neuron-astrocyte interaction as a key contributor to hyperexcitability in several pathologies.
Keywords: cell biology; neuroscience.
Crown Copyright © 2025 Published by Elsevier Inc.
Conflict of interest statement
The authors declare competing interests.
Figures



References
-
- Bae Y.C., Park K.P., Yoshida A., Nakagawa S., Kurata S., Chen K., Takemura M., Shigenaga Y. Identification of gamma-aminobutyric acid-immunoreactive axon endings associated with mesencephalic periodontal afferent terminals and morphometry of the two types of terminals in the cat supratrigeminal nucleus. J. Comp. Neurol. 1997;389:127–138. doi: 10.1002/(sici)1096-9861(19971208)389:1<127::aid-cne9>3.0.co;2-4. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

