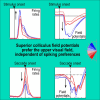
Superior colliculus peri-saccadic field potentials are dominated by a visual sensory preference for the upper visual field
- PMID: 40104053
- PMCID: PMC11914513
- DOI: 10.1016/j.isci.2025.112021
Superior colliculus peri-saccadic field potentials are dominated by a visual sensory preference for the upper visual field
Abstract
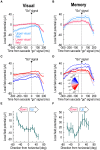
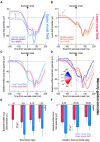
The primate superior colliculus (SC) plays important sensory, cognitive, and motor processing roles. Among its properties, the SC has clear visual field asymmetries: visual responses are stronger in the upper visual field representation, whereas saccade-related motor bursts are weaker. Here, I asked whether peri-saccadic SC network activity can still reflect the SC's visual sensitivity asymmetry, thus supporting recent evidence of sensory-related signals embedded within the SC's motor bursts. I analyzed collicular peri-saccadic local field potential (LFP) modulations and found them to be much stronger in the upper visual field, despite the weaker motor bursts. This effect persisted even with saccades toward a blank, suggesting an importance of visual field location. I also found that engaging working memory during saccade preparation differentially modulated the SC's LFP's, again with a dichotomous upper/lower visual field asymmetry. I conclude that the SC network possesses a clear sensory signal at the time of saccade generation.
Keywords: Cognitive neuroscience; Neuroscience; Sensory neuroscience.
© 2025 The Author(s).
Conflict of interest statement
The author declares no competing interests.
Figures




References
-
- Robinson D.A. Eye movements evoked by collicular stimulation in the alert monkey. Vis. Res. 1972;12:1795–1808. - PubMed
-
- Munoz D.P., Wurtz R.H. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J. Neurophysiol. 1995;73:2313–2333. - PubMed
-
- Munoz D.P., Wurtz R.H. Saccade-related activity in monkey superior colliculus. II. Spread of activity during saccades. J. Neurophysiol. 1995;73:2334–2348. - PubMed
LinkOut - more resources
Full Text Sources

