Islet single-cell transcriptomic profiling during obesity-induced beta cell expansion in female mice
- PMID: 40104055
- PMCID: PMC11914824
- DOI: 10.1016/j.isci.2025.112031
Islet single-cell transcriptomic profiling during obesity-induced beta cell expansion in female mice
Abstract
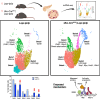
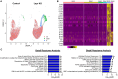
Targeting beta cell proliferation is an appealing approach to restore glucose control in type 1 diabetes. However, the underlying mechanisms of beta cell proliferation remain incompletely understood, limiting identification of new therapeutic targets. Obesity is a naturally occurring process that potently induces human and rodent beta cell replication, representing an ideal model to study mechanisms of beta cell proliferation. We showed previously acute whole-body Lepr gene deletion in adult mice induces obesity and massive beta cell expansion. Here, using single-cell transcriptomics with female Lepr KO islets, we identified distinct populations of beta cells undergoing unfolded protein response (UPR), stress resolution, and cell cycle progression. Lepr KO beta cells undergoing UPR markedly increased chaperone protein, ribosomal biogenesis, and cell cycle transcriptional programs that were enriched for Xbp1 and Myc target genes. Our findings suggest a coordinated transcriptional mechanism involving Xbp1 and Myc to alleviate UPR and stimulate beta cell proliferation in obese female mice.
Keywords: Biological sciences; Cell biology; Functional aspects of cell biology; Specialized functions of cells.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The extra-islet pancreas supports autoimmunity in human type 1 diabetes.Elife. 2025 Apr 15;13:RP100535. doi: 10.7554/eLife.100535. Elife. 2025. PMID: 40232951 Free PMC article.
-
Exploring lncRNAs associated with human pancreatic islet cell death induced by transfer of adoptive lymphocytes in a humanized mouse model.Front Endocrinol (Lausanne). 2023 Nov 1;14:1244688. doi: 10.3389/fendo.2023.1244688. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38027148 Free PMC article.
-
METRNL represses beta-to-alpha cell trans-differentiation to maintain beta cell function under diabetic metabolic stress in mice.Diabetologia. 2025 Aug;68(8):1769-1788. doi: 10.1007/s00125-025-06459-7. Epub 2025 Jun 10. Diabetologia. 2025. PMID: 40495021
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
Hormonal contraceptives for contraception in overweight or obese women.Cochrane Database Syst Rev. 2016 Aug 18;2016(8):CD008452. doi: 10.1002/14651858.CD008452.pub4. Cochrane Database Syst Rev. 2016. PMID: 27537097 Free PMC article.
References
-
- Smukler S.R., Arntfield M.E., Razavi R., Bikopoulos G., Karpowicz P., Seaberg R., Dai F., Lee S., Ahrens R., Fraser P.E., et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8:281–293. doi: 10.1016/j.stem.2011.01.015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

