Observational studies of exposure to tobacco and nicotine products: Best practices for maximizing statistical precision and accuracy
- PMID: 40104063
- PMCID: PMC11915159
- DOI: 10.1016/j.isci.2025.111985
Observational studies of exposure to tobacco and nicotine products: Best practices for maximizing statistical precision and accuracy
Abstract
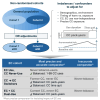
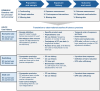
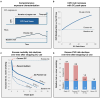
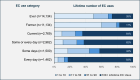
Non-randomized observational studies can track risk-induction and -reduction associated with real-world use of non-combusted nicotine and tobacco products. The objective of this analysis was to evaluate the precision and accuracy of recent studies and to identify opportunities for further optimizing future study designs. The ROBINS framework for minimizing statistical bias was translated to specific considerations that spanned the selection and quantification of cohorts, exposure, and outcomes. These principles were then considered within the context of a recent comprehensive meta-analysis, representing 107 observational studies, which evaluated the effects of using electronic cigarettes (ECs), combusted cigarettes (CCs) and dual use of both. The meta-analysis had previously reported the relative risk from all-sources, including tobacco use and non-tobacco use. We now report the product use-specific risk associated with displacing CCs with ECs indicated from the primary references, along with observations regarding the precision of characterization of CC and EC exposure in the cited studies.
Keywords: Health sciences; Medicine; Research methodology social sciences.
© 2025 The Author(s).
Conflict of interest statement
G.C. is a salaried employee of Rose Research Center (RRC), an independent contract research organization that performs studies pertaining to smoking cessation and tobacco harm reduction. Research support for other projects: National Institute on Drug Abuse; Global Action to End Smoking, Inc. (formerly Foundation for a Smoke-Free World, Inc.), a US nonprofit 501(c)(3) private foundation; Nicotine BRST LLC; JUUL Labs; Altria; Embera Neurotherapeutics, Inc.; Otsuka Pharmaceutical; Swedish Match, Philip Morris International. G.C. was previously a Principal Scientist at JUUL Labs. He also was employed at Nektar Therapeutics, whose pipeline included an inhaled NRT. Stock holdings in Qnovia, a developer of an inhaled NRT, and JUUL Labs. This review was not funded nor commissioned by any of these non-RRC entities. S.C. has no declarations of interest to declare, beyond acknowledgment of NIH and CTP funding.
Figures



References
-
- American Lung Association Tobacco Facts | State of Tobacco Control. 2024. https://www.lung.org/research/sotc/facts
-
- WHO WHO Report on the Global Tobacco Epidemic, 2023: Protect People From Tobacco Smoke. World Health Organization. 2023. https://www.who.int/publications/i/item/9789240077164
-
- Erhabor J., Boakye E., Obisesan O., Osei A.D., Tasdighi E., Mirbolouk H., DeFilippis A.P., Stokes A.C., Hirsch G.A., Benjamin E.J., et al. E-Cigarette Use Among US Adults in the 2021 Behavioral Risk Factor Surveillance System Survey. JAMA Netw. Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.40859. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

