Immune-driven gene expression loss following intramuscular AAV delivery to non-human primates is only transient
- PMID: 40104151
- PMCID: PMC11914520
- DOI: 10.1016/j.omtm.2025.101409
Immune-driven gene expression loss following intramuscular AAV delivery to non-human primates is only transient
Abstract
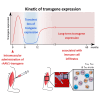
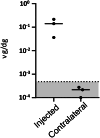
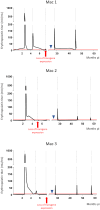
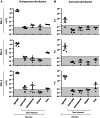
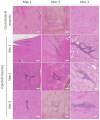
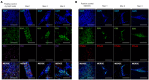
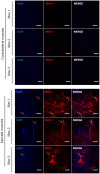
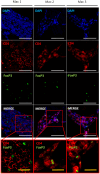
Recombinant adeno-associated virus (rAAV) vectors stand out as highly promising for in vivo gene transfer, particularly in targeting the skeletal muscle for treating muscular genetic diseases or secreting therapeutic factors. Despite the simplicity and efficacy of the established intramuscular (IM) route, it has been often associated with an immune-induced rapid loss of transgene expression, in particular in large animal models, and generally considered irreversible as a consequence of a cytotoxic elimination of transduced cells. Here, we report in a non-human primate model that transgene expression loss after IM delivery of an rAAV1 expressing an immunogenic protein is only transient, with the re-expression of the transgene lasting up to 5 years post-injection. We show that the recovery of transgene expression is due to persisting viral genomes in the injected muscles despite the detection of peripheral anti-transgene cellular immunity. Persisting genomes were observed in the presence of infiltrated mononuclear CD8 and CD4 T lymphocytes, among which we were able to detect FoxP3+ regulatory cells. This is to our knowledge the first report of a transient immune-mediated loss of gene expression in a large animal model after rAAV delivery that should shed new light on the issue of rAAV vector immunogenicity.
Keywords: AAV; Tregs; gene transfer; immune response; immunomodulation; intramuscular; non-human primate; transitory loss.
© 2025 Published by Elsevier Inc. on behalf of The American Society of Gene and Cell Therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

