Therapeutic potential of fecal microbiota transplantation in colorectal cancer based on gut microbiota regulation: from pathogenesis to efficacy
- PMID: 40104324
- PMCID: PMC11915259
- DOI: 10.1177/17562848251327167
Therapeutic potential of fecal microbiota transplantation in colorectal cancer based on gut microbiota regulation: from pathogenesis to efficacy
Abstract
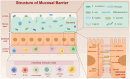
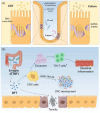
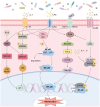
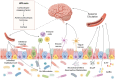
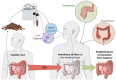
Colorectal cancer (CRC) remains a leading cause of cancer-related deaths worldwide, with its progression intricately linked to gut microbiota dysbiosis. Disruptions in microbial homeostasis contribute to tumor initiation, immune suppression, and inflammation, establishing the microbiota as a key therapeutic target. Fecal microbiota transplantation (FMT) has emerged as a transformative approach to restore microbial balance, enhance immune responses, and reshape the tumor microenvironment. This review explores the mechanisms underlying FMT's therapeutic potential, evaluates its advantages over other microbiota-based interventions, and addresses challenges such as donor selection, safety concerns, and treatment standardization. Looking forward, the integration of FMT into personalized CRC therapies requires robust clinical trials and the identification of predictive biomarkers to optimize its efficacy and safety.
Keywords: colorectal cancer; fecal microbiota transplantation; gut microbiome; microbiota–gut–brain.
Plain language summary
FMT in CRC: microbiota regulation to therapy Colorectal cancer (CRC) progression is tied to gut microbiota dysbiosis. Fecal microbiota transplantation (FMT) restores balance and boosts immunity, offering promise for personalized CRC therapies with further clinical validation.
© The Author(s), 2025.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Fecal Microbiota Transplantation: Insights into Colon Carcinogenesis and Immune Regulation.J Clin Med. 2024 Nov 1;13(21):6578. doi: 10.3390/jcm13216578. J Clin Med. 2024. PMID: 39518717 Free PMC article. Review.
-
From chaos to order: optimizing fecal microbiota transplantation for enhanced immune checkpoint inhibitors efficacy.Gut Microbes. 2025 Dec;17(1):2452277. doi: 10.1080/19490976.2025.2452277. Epub 2025 Jan 18. Gut Microbes. 2025. PMID: 39826104 Review.
-
The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection.Therap Adv Gastroenterol. 2022 Nov 18;15:17562848221134396. doi: 10.1177/17562848221134396. eCollection 2022. Therap Adv Gastroenterol. 2022. PMID: 36425405 Free PMC article. Review.
-
Fecal microbiota transplantation inhibits colorectal cancer progression: Reversing intestinal microbial dysbiosis to enhance anti-cancer immune responses.Front Microbiol. 2023 Apr 18;14:1126808. doi: 10.3389/fmicb.2023.1126808. eCollection 2023. Front Microbiol. 2023. PMID: 37143538 Free PMC article.
-
Exploring the Potential of Fecal Microbiota Transplantation as a Therapy in Tuberculosis and Inflammatory Bowel Disease.Pathogens. 2023 Sep 9;12(9):1149. doi: 10.3390/pathogens12091149. Pathogens. 2023. PMID: 37764957 Free PMC article. Review.
References
-
- Siegel RL, Wagle NS, Cercek A, et al.. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023; 73(3): 233–254. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

