Serum-free Quality and Quantity Control Culture Improves the Angiogenic Potential of Peripheral Blood Mononuclear Cells Harvested from Patients with Connective Tissue Diseases
- PMID: 40104559
- PMCID: PMC11912996
- DOI: 10.53045/jprs.2022-0050
Serum-free Quality and Quantity Control Culture Improves the Angiogenic Potential of Peripheral Blood Mononuclear Cells Harvested from Patients with Connective Tissue Diseases
Abstract
Objectives: The number and quality of endothelial progenitor cells decrease in patients with connective tissue diseases. This limits the efficacy of mononuclear cell therapy for ischemic ulcers associated with connective tissue diseases. To overcome these problems, we developed a serum-free quality and quantity control culture method that potentially improves the function of endothelial progenitor cells and expands their numbers. Here, we show the effect of quality and quantity control culture on mononuclear cells from patients with connective tissue diseases.
Methods: Peripheral blood mononuclear cells were isolated from C57BL/6JJmsSlc-lpr/lpr mice with systemic lupus erythematosus, patients with connective tissue diseases, and healthy volunteers. Mononuclear cells were cultured using the quality and quantity control culture method, and the number of endothelial progenitor cells was analyzed using flow cytometry, an endothelial progenitor cell culture assay, and an endothelial progenitor cell colony-forming assay. Flow cytometry was also used to examine mononuclear cell subpopulations. A human umbilical vein endothelial cell tube-forming assay was used to examine the function of quality and quantity control cultured mononuclear cells.
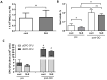
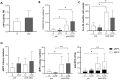
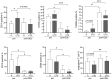
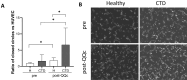
Results: Mice with systemic lupus erythematosus showed a significantly lower number of endothelial progenitor cells, which increased to the same levels as those of the control mice after quality and quantity control culture. In humans, the numbers of endothelial progenitor cells and M2 macrophages were significantly increased and the number of proinflammatory cells was decreased after quality and quantity control culture in both healthy volunteers and patients with connective tissue diseases. The human umbilical vein endothelial cell tube formation assay showed higher angiogenic potential in quality and quantity control cultured mononuclear cells from patients with connective tissue diseases than that in quality and quantity control cultured mononuclear cells from healthy controls.
Conclusions: Our study suggests that the quality and quantity control culture method is effective in recovering the angiogenic ability of mononuclear cells from patients with connective tissue diseases.
Keywords: MNC-QQ cell; cell therapy; connective tissue disease; peripheral blood mononuclear cell; vasculogenesis.
© 2024 The Japan Society of Plastic and Reconstructive Surgery.
Conflict of interest statement
Conflicts of Interest: Rica Tanaka was the Chief Executive Officer of ReEir, Inc.
Figures
References
-
- Asahara T, Murohara T, Sullivan, et al. . Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997 Feb;275(5302):964-7. - PubMed
-
- Qadura M, Terenzi DC, Verma S, et al. . Concise review: cell therapy for critical limb ischemia: an integrated review of preclinical and clinical studies. Stem Cells. 2018 Feb;36(2):161-71. - PubMed
-
- Wahid FSA, Ismail NA, Wan Jamaludin WF, et al. . Efficacy and safety of autologous cell-based therapy in patients with no-option critical limb ischaemia: a meta-analysis. Curr Stem Cell Res Ther. 2018;13(4):265-83. - PubMed
-
- Tanaka R, Wada M, Kwon SM, et al. . The effects of flap ischemia on normal and diabetic progenitor cell function. Plast Reconstr Surg. 2008 Jun;121(6):1929-42. - PubMed
-
- Tsukada S, Masuda H, Jung SY, et al. . Impaired development and dysfunction of endothelial progenitor cells in type 2 diabetic mice. Diabetes Metab. 2017 Apr;43(2):154-62. - PubMed

