Neck Dissection and Survival Among Head and Neck Cancer Patients Undergoing Adjuvant Immunotherapy
- PMID: 40104563
- PMCID: PMC11915686
- DOI: 10.1002/lio2.70120
Neck Dissection and Survival Among Head and Neck Cancer Patients Undergoing Adjuvant Immunotherapy
Abstract
Background: Some studies suggest that neck dissection (ND) should be avoided in candidates for immunotherapy because lymph nodes are primary sites for immunotherapy activation. Our study investigates ND utilization and associated differences in overall survival (OS) among patients with head and neck cancer (HNC) undergoing adjuvant immunotherapy.
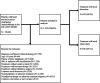
Methods: The 2013-2018 National Cancer Database was retrospectively reviewed for patients with HNC undergoing surgery with curative intent, and adjuvant immunotherapy. Multivariable binary logistic and Cox regression models adjusted for patient demographics, clinicopathologic features, and treatment.
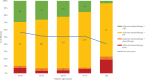
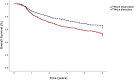
Results: Of 1335 patients satisfying inclusion criteria, 679 (50.9%) patients underwent ND: 94 (13.8%) had pN0, 109 (16.1%) had pN1, 411 (60.5%) had pN2, 60 (8.8%) had pN3, and 5 (0.7%) had pNx classification. On multivariable binary logistic regression, academic treatment facility, cT4, and cN1-3 classification were associated with higher odds of undergoing ND (p < 0.05); salivary, sinonasal, oropharyngeal, hypopharyngeal, and laryngeal primary sites were associated with decreased odds (p < 0.05). Compared with those undergoing neck observation, patients undergoing ND had worse OS (49.4% vs. 61.5%, p < 0.001) on Kaplan-Meier but not multivariable Cox (adjusted hazard ratio [aHR] 1.00, 95% confidence interval [CI] 0.82-1.24, p = 0.968) regression. Compared with adjuvant immunotherapy alone, the addition of radiotherapy (aHR 0.64, 95% CI 0.44-0.93) and chemoradiotherapy (aHR 0.56, 95% CI 0.37-0.86) were associated with higher OS (p < 0.025).
Conclusion: ND was utilized in approximately 51% of patients with HNC undergoing adjuvant immunotherapy. ND was not associated with worse OS, possibly related to the high rate of pN1-3 classification.
Level of evidence: 4.
Keywords: National Cancer Database; head and neck cancer; immunotherapy; radiotherapy; survival.
© 2025 The Author(s). Laryngoscope Investigative Otolaryngology published by Wiley Periodicals LLC on behalf of The Triological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Extranodal extension in laryngeal squamous cell carcinoma.Laryngoscope Investig Otolaryngol. 2024 Mar 25;9(2):e1232. doi: 10.1002/lio2.1232. eCollection 2024 Apr. Laryngoscope Investig Otolaryngol. 2024. PMID: 38529341 Free PMC article.
-
Choice of Adjuvant Radiotherapy Facility in Major Salivary Gland Cancer.Laryngoscope. 2024 Aug;134(8):3620-3632. doi: 10.1002/lary.31352. Epub 2024 Feb 24. Laryngoscope. 2024. PMID: 38400788
-
Choice of Adjuvant Radiotherapy Facility in Sinonasal Squamous Cell Carcinoma.Laryngoscope. 2025 Feb;135(2):705-715. doi: 10.1002/lary.31794. Epub 2024 Sep 24. Laryngoscope. 2025. PMID: 39315470 Free PMC article.
-
Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment.Cochrane Database Syst Rev. 2018 Dec 24;12(12):CD006205. doi: 10.1002/14651858.CD006205.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Aug 31;8:CD006205. doi: 10.1002/14651858.CD006205.pub5. PMID: 30582609 Free PMC article. Updated.
-
Survival outcomes for head and neck cancer patients with N3 cervical nodal metastases.Clin Otolaryngol. 2020 May;45(3):342-349. doi: 10.1111/coa.13501. Epub 2020 Feb 20. Clin Otolaryngol. 2020. PMID: 31869000
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
