Inhibitory effects of carvacrol on glucansucrase from Streptococcus mutans and salivary α-amylase: in silico and in vitro studies
- PMID: 40104578
- PMCID: PMC11913353
- DOI: 10.55730/1300-0152.2727
Inhibitory effects of carvacrol on glucansucrase from Streptococcus mutans and salivary α-amylase: in silico and in vitro studies
Abstract
Background/aim: Streptococcus mutans produces glucansucrase, an enzyme that converts sucrose into lactic acid, which lowers the pH in the oral environment and leads to tooth enamel demineralization, a key factor in dental caries. Additionally, glucansucrase facilitates the formation of extracellular polysaccharides, which promote bacterial adhesion to tooth surfaces. This study investigates the inhibitory effects of carvacrol, a natural compound, on glucansucrase activity both in vitro and in silico.
Materials and methods: Glucansucrase enzyme was purified from S. mutans. The inhibitory effects of carvacrol against glucansucrase enzyme were investigated both in vitro and in silico.
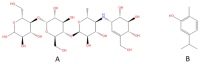
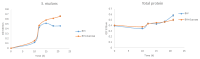
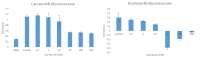
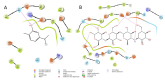
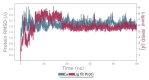
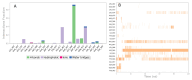
Results: In the presence of 50 mM carvacrol, glucansucrase and salivary amylase activities were reduced by 51.25% and 14.85%, respectively. Carvacrol did not significantly inhibit (4.73%) the salivary amylase enzyme at 10 mM. Glucansucrase activity decreased by 51.63% in the presence of 10 mM acarbose, which was used as a positive control in glucansucrase enzyme studies. Acarbose inhibited salivary amylase with 82.54% loss of enzyme activity in the presence of 1 mM acarbose. The docking score obtained for carvacrol was -5.262 kcal/mol, while that obtained for acarbose was -6.084 kcal/mol. We carried out molecular dynamics simulation studies for 100 ns to determine the stability of carvacrol in the active site of the protein. Carvacrol demonstrated stable binding to glucansucrase with hydrogen bonds and interactions at key residues (ASP477, GLN960, and ASP909), confirmed by molecular dynamics simulations. Carvacrol remained stable between 16 and 100 ns.
Conclusion: Carvacrol selectively inhibits glucansucrase without significantly affecting salivary amylase, making it a more targeted inhibitor compared to acarbose, which inhibits both enzymes. Docking studies indicated that while carvacrol has a lower binding affinity than acarbose, its stable interaction with the enzyme suggests sustained inhibitory action. These findings highlight carvacrol as a promising natural compound for preventing dental caries, offering a more selective alternative to traditional inhibitors. Further in vivo studies are necessary to assess its therapeutic efficacy and safety in clinical applications for oral health.
Keywords: Carvacrol; Streptococcus mutans; amylase; biofilm; glucansucrase.
© TÜBİTAK.
Conflict of interest statement
Conflicts of interest: The authors declare that there are no conflicts of interest.
Figures
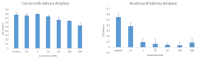
Similar articles
-
Inhibitory potential of EGCG on Streptococcus mutans biofilm: A new approach to prevent Cariogenesis.Microb Pathog. 2020 Jun;143:104129. doi: 10.1016/j.micpath.2020.104129. Epub 2020 Mar 10. Microb Pathog. 2020. PMID: 32169491
-
Molecular insights into antibiofilm inhibitors of Streptococcus mutans glucosyltransferases through in silico approaches.Sci Rep. 2025 Apr 23;15(1):14160. doi: 10.1038/s41598-025-98927-8. Sci Rep. 2025. PMID: 40269071 Free PMC article.
-
Six Spain Thymus essential oils composition analysis and their in vitro and in silico study against Streptococcus mutans.BMC Complement Med Ther. 2023 Apr 5;23(1):106. doi: 10.1186/s12906-023-03928-7. BMC Complement Med Ther. 2023. PMID: 37020229 Free PMC article.
-
Natural compounds: new therapeutic approach for inhibition of Streptococcus mutans and dental caries.Front Pharmacol. 2025 Apr 1;16:1548117. doi: 10.3389/fphar.2025.1548117. eCollection 2025. Front Pharmacol. 2025. PMID: 40235544 Free PMC article. Review.
-
Salivary alpha-amylase: role in dental plaque and caries formation.Crit Rev Oral Biol Med. 1993;4(3-4):301-7. doi: 10.1177/10454411930040030701. Crit Rev Oral Biol Med. 1993. PMID: 8373987 Review.
References
-
- Aazza S, El-Guendouz S, Miguel MG, Antunes MD, Faleiro ML, et al. Antioxidant, anti-inflammatory and anti-hyperglycaemic activities of essential oils from Thymbra capitata, Thymus albicans, Thymus caespititius, Thymus carnosus, Thymus lotocephalus and Thymus mastichina from Portugal. Natural Product Communications. 2016;11(7):1029–1038. doi: 10.1177/1934578X1601100739. - DOI - PubMed
-
- Amaral VC, Santos PR, da Silva AF, dos Santos AR, Machinski Jr M, et al. Effect of carvacrol and thymol on Salmonella spp. biofilms on polypropylene. International Journal of Food Science & Technology. 2015;50(12):2639–2643. doi: 10.1111/ijfs.12934. - DOI
-
- Bowers KJ, Chow DE, Xu H, Dror RO, Eastwood MP, et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. Proceedings of the 2006 ACM/IEEE Conference on Supercomputing; New York, NY, USA: Association for Computing Machinery; 2006. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
