Understanding dysbiosis and resilience in the human gut microbiome: biomarkers, interventions, and challenges
- PMID: 40104586
- PMCID: PMC11913848
- DOI: 10.3389/fmicb.2025.1559521
Understanding dysbiosis and resilience in the human gut microbiome: biomarkers, interventions, and challenges
Abstract
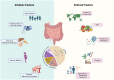
The healthy gut microbiome is important in maintaining health and preventing various chronic and metabolic diseases through interactions with the host via different gut-organ axes, such as the gut-brain, gut-liver, gut-immune, and gut-lung axes. The human gut microbiome is relatively stable, yet can be influenced by numerous factors, such as diet, infections, chronic diseases, and medications which may disrupt its composition and function. Therefore, microbial resilience is suggested as one of the key characteristics of a healthy gut microbiome in humans. However, our understanding of its definition and indicators remains unclear due to insufficient experimental data. Here, we review the impact of key drivers including intrinsic and extrinsic factors such as diet and antibiotics on the human gut microbiome. Additionally, we discuss the concept of a resilient gut microbiome and highlight potential biomarkers including diversity indices and some bacterial taxa as recovery-associated bacteria, resistance genes, antimicrobial peptides, and functional flexibility. These biomarkers can facilitate the identification and prediction of healthy and resilient microbiomes, particularly in precision medicine, through diagnostic tools or machine learning approaches especially after antimicrobial medications that may cause stable dysbiosis. Furthermore, we review current nutrition intervention strategies to maximize microbial resilience, the challenges in investigating microbiome resilience, and future directions in this field of research.
Keywords: antibiotics; biomarkers; dysbiosis; human gut microbiome; microbiome recovery; perturbation; resilient gut microbiome.
Copyright © 2025 Safarchi, Al-Qadami, Tran and Conlon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Microbiome Resilience despite a Profound Loss of Minority Microbiota following Clindamycin Challenge in Humanized Gnotobiotic Mice.Microbiol Spectr. 2022 Feb 23;10(1):e0196021. doi: 10.1128/spectrum.01960-21. Epub 2022 Jan 12. Microbiol Spectr. 2022. PMID: 35019780 Free PMC article.
-
Gut Microbiota Resilience: Definition, Link to Health and Strategies for Intervention.Front Microbiol. 2020 Sep 15;11:572921. doi: 10.3389/fmicb.2020.572921. eCollection 2020. Front Microbiol. 2020. PMID: 33042082 Free PMC article. Review.
-
The current findings on the gut-liver axis and the molecular basis of NAFLD/NASH associated with gut microbiome dysbiosis.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 9. doi: 10.1007/s00210-025-04069-z. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40202676 Review.
-
Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health.Gut. 2021 Mar;70(3):595-605. doi: 10.1136/gutjnl-2020-321747. Epub 2020 Oct 13. Gut. 2021. PMID: 33051190 Review.
-
Establishing What Constitutes a Healthy Human Gut Microbiome: State of the Science, Regulatory Considerations, and Future Directions.J Nutr. 2019 Nov 1;149(11):1882-1895. doi: 10.1093/jn/nxz154. J Nutr. 2019. PMID: 31373365 Free PMC article. Review.
Cited by
-
Assessing the Antibiotic Resistance in Food Lactic Acid Bacteria: Risks in the Era of Widespread Probiotic Use.Food Sci Nutr. 2025 Jul 31;13(8):e70740. doi: 10.1002/fsn3.70740. eCollection 2025 Aug. Food Sci Nutr. 2025. PMID: 40755505 Free PMC article. Review.
-
Characterizing Gene-Level Adaptations in the Gut Microbiome During Viral Infections: The Role of a Fucoidan-Rich Extract.Genes (Basel). 2025 Jun 26;16(7):740. doi: 10.3390/genes16070740. Genes (Basel). 2025. PMID: 40725397 Free PMC article.
References
-
- Acharya C., Betrapally N. S., Gillevet P. M., Sterling R. K., Akbarali H., White M. B., et al. . (2017). Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment. Pharmacol. Ther. 45, 319–331. doi: 10.1111/apt.13858, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

