Recombinant Expression of a Ready-to-Use EGF Variant Equipped With a Single Conjugation Site for Click-Chemistry
- PMID: 40104837
- PMCID: PMC11913717
- DOI: 10.1002/elsc.70015
Recombinant Expression of a Ready-to-Use EGF Variant Equipped With a Single Conjugation Site for Click-Chemistry
Abstract
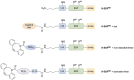
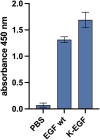
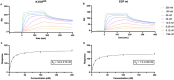
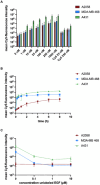
The epidermal growth factor (EGF) receptor is commonly targeted in cancer therapy because it is overexpressed in many malignant cells. However, a general problem is to couple the targeting moieties and the drug molecules in a way that results in a homogeneous product. Here, we overcome this issue by engineering a variant of EGF with a single conjugation site for coupling virtually any payload. The recombinant EGF variant K-EGFRR was expressed in E. coli Rosetta with a 4-6 mg/L yield. To confirm the accessibility of the introduced functional group, the ligand was equipped with a sulfo-cyanine dye with a loading of 0.65 dye per ligand, which enables tracking in vitro. The kinetics and affinity of ligand-receptor interaction were evaluated by enzyme-linked immunosorbent assay and surface plasmon resonance. The affinity of K-EGFRR was slightly higher when compared to the wild-type EGF (K D: 5.9 vs. 7.3 nM). Moreover, the ligand-receptor interaction and uptake in a cellular context were evaluated by flow cytometry and quantitative high-content imaging. Importantly, by attaching heterobifunctional polyethylene glycol linkers, we allowed orthogonal click-conjugation of the ligand to any payload of choice, making K-EGFRR an ideal candidate for targeted drug delivery.
Keywords: click‐chemistry; epidermal growth factor; ligand variant; single conjugation site; targeted therapy.
© 2025 The Author(s). Engineering in Life Sciences published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures


Similar articles
-
EGF receptor-targeting peptide conjugate incorporating a near-IR fluorescent dye and a novel 1,4,7-triazacyclononane-based (64)Cu(II) chelator assembled via click chemistry.Bioconjug Chem. 2014 May 21;25(5):1011-22. doi: 10.1021/bc5001388. Epub 2014 Apr 30. Bioconjug Chem. 2014. PMID: 24758412
-
Tethering Growth Factors to Collagen Surfaces Using Copper-Free Click Chemistry: Surface Characterization and in Vitro Biological Response.ACS Appl Mater Interfaces. 2017 Jul 19;9(28):23389-23399. doi: 10.1021/acsami.7b05262. Epub 2017 Jul 6. ACS Appl Mater Interfaces. 2017. PMID: 28598594
-
An analytical approach to the measurement of equilibrium binding constants: application to EGF binding to EGF receptors in intact cells measured by flow cytometry.Biochemistry. 2001 May 22;40(20):6142-54. doi: 10.1021/bi002817a. Biochemistry. 2001. PMID: 11352752
-
Epidermal growth factor-PEG functionalized PAMAM-pentaethylenehexamine dendron for targeted gene delivery produced by click chemistry.Biomacromolecules. 2011 Jun 13;12(6):2039-47. doi: 10.1021/bm101464n. Epub 2011 Apr 27. Biomacromolecules. 2011. PMID: 21491906
-
Surface plasmon resonance spectroscopy for characterisation of membrane protein-ligand interactions and its potential for drug discovery.Biochim Biophys Acta. 2014 Jan;1838(1 Pt A):43-55. doi: 10.1016/j.bbamem.2013.04.028. Epub 2013 May 9. Biochim Biophys Acta. 2014. PMID: 23665295 Review.
References
-
- Aslam M., Naveed S., Ahmad A., et al., “Side Effects of Chemotherapy in Cancer Patients and Evaluation of Patients Opinion About Starvation Based Differential Chemotherapy,” Journal of Cancer Therapy 5 (2014): 817–822.
-
- Yewale C., Baradia D., Vhora I., Patil S., and Misra A., “Epidermal Growth Factor Receptor Targeting in Cancer: A Review of Trends and Strategies,” Biomaterials 34 (2013): 8690–8707. - PubMed
-
- Yarden Y., “The EGFR Family and Its Ligands in Human Cancer: Signalling Mechanisms and Therapeutic Opportunities,” European Journal of Cancer 37, no. Suppl4 (2001): S3–S8. - PubMed
LinkOut - more resources
Full Text Sources
