Pharmacokinetics of Milbemycin Oxime in Pekingese Dogs after Single Oral and Intravenous Administration
- PMID: 40104878
- PMCID: PMC11920723
- DOI: 10.1002/vms3.70312
Pharmacokinetics of Milbemycin Oxime in Pekingese Dogs after Single Oral and Intravenous Administration
Abstract
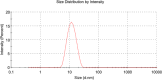
Objective: This study aimed to characterize the pharmacokinetic profiles of milbemycin oxime in Pekingese dogs following a single oral (PO) and intravenous (IV) dose. Six clinically healthy Pekingese dogs, with an average body weight (BW) of 4.75 kg, were included. Each dog received an IV injection of milbemycin oxime solution and PO doses of both milbemycin oxime tablets and nanoemulsion, all administered at 1 mg/kg BW.
Methods: Blood samples (∼0.6 mL) were collected at various time points, and milbemycin oxime concentrations were measured using a validated high-performance liquid chromatography (HPLC) method with ultraviolet (UV) detection. Pharmacokinetic parameters were obtained through non-compartmental analysis (NCA) using WinNonLin software.
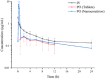
Results: Oral administration of milbemycin oxime tablets resulted in a peak concentration (Cmax) of 0.33 ± 0.07 µg/mL at 2.47 ± 1.90 h, with a mean residence time (MRT) of 21.96 ± 14.43 h and an absolute bioavailability of 51.44% ± 21.76%. In contrast, the nanoemulsion achieved a significantly higher Cmax of 8.87 ± 1.88 µg/mL, with a much quicker time to peak concentration (Tmax) at 0.33 ± 0.13 h, an MRT of 21.74 ± 18.21 h, and an absolute bioavailability of 99.26% ± 12.14%. After IV administration, total clearance (Cl) and steady-state volume of distribution (VSS) were 0.13 ± 0.06 mL/kg/h and 2.36 ± 0.73 mL/kg, respectively.
Conclusions: These findings demonstrate that the milbemycin oxime nanoemulsion is absorbed more rapidly and completely, with significantly higher bioavailability than the tablet form. This suggests that the nanoemulsion could effectively overcome the issues of poor diffusion and low bioavailability associated with tablet formulations, positioning it as a promising alternative to traditional milbemycin oxime tablets.
Keywords: Pekingese dogs; bioavailability; milbemycin oxime; nanoemulsion; pharmacokinetics.
© 2025 The Author(s). Veterinary Medicine and Science published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Pharmacokinetic profile of a single intramuscular or oral dose of meloxicam in red kangaroos (Osphranter rufus).Am J Vet Res. 2025 Mar 21;86(6):ajvr.24.10.0326. doi: 10.2460/ajvr.24.10.0326. Print 2025 Jun 1. Am J Vet Res. 2025. PMID: 40118024
-
Single-Dose, Intravenous, and Oral Pharmacokinetics of Isavuconazole in Dogs.J Vet Pharmacol Ther. 2025 Jul;48(4):234-240. doi: 10.1111/jvp.13510. Epub 2025 Apr 8. J Vet Pharmacol Ther. 2025. PMID: 40200720 Free PMC article.
-
Pharmacokinetics, bioavailability and plasma protein binding of tolfenamic acid in rainbow trout (Oncorhynchus mykiss).Vet Med Sci. 2024 Jul;10(4):e1533. doi: 10.1002/vms3.1533. Vet Med Sci. 2024. PMID: 38952278 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Impact of milbemycin oxime on fluconazole resistance in Candida auris.JAC Antimicrob Resist. 2025 Apr 12;7(2):dlaf060. doi: 10.1093/jacamr/dlaf060. eCollection 2025 Apr. JAC Antimicrob Resist. 2025. PMID: 40224361 Free PMC article.
References
-
- Blagburn, B. L. , Hendrix C. M., Lindsay D. S., Vaughan J. L., Hepler D. I., and Wright J. C.. 1992. “Efficacy of Milbemycin Oxime Against Naturally Acquired or Experimentally Induced Ancylostoma spp and Trichuris vulpis Infections in Dogs.” American Journal of Veterinary Research 53, no. 4: 513–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

