Malignant Cells Beyond the Tumor Core: The Non-Negligible Factor to Overcome the Refractory of Glioblastoma
- PMID: 40104956
- PMCID: PMC11920816
- DOI: 10.1111/cns.70333
Malignant Cells Beyond the Tumor Core: The Non-Negligible Factor to Overcome the Refractory of Glioblastoma
Abstract
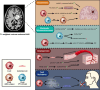
Background: Glioblastoma (GBM) is one of the most aggressive primary brain tumors in adults. Over 95% of GBM patients experience recurrence in the peritumoral brain tissue or distant regions, indicating the presence of critical factors in these areas that drive tumor recurrence. Current clinical treatments primarily focus on tumor cells from the tumor core (TC), while the role of neoplastic cells beyond the TC has been largely neglected.
Methods: We conducted a comprehensive review of existing literature and studies on GBM, focusing on the identification and characterization of questionable cells (Q cells). Advanced imaging techniques, such as diffusion tensor imaging (DTI), magnetic resonance spectroscopy (MRS), and positron emission tomography (PET), were utilized to identify Q cells beyond the tumor core. We also analyzed the functional properties, cellular microenvironment, and physical characteristics of Q cells, as well as their implications for surgical resection.
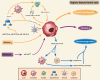
Results: Our review revealed that Q cells exhibit unique functional attributes, including enhanced invasiveness, metabolic adaptations, and resistance mechanisms. These cells reside in a distinct cellular microenvironment and are influenced by physical properties such as solid stress and stiffness. Advanced imaging techniques have improved the identification of Q cells, enabling more precise surgical resection. Targeting Q cells in therapeutic strategies could significantly reduce the risk of GBM recurrence.
Conclusion: The presence of Q cells in the peritumoral brain zone (PBZ) and beyond is a critical factor in GBM recurrence. Current treatments, which primarily target tumor cells in the TC, are insufficient to prevent recurrence due to the neglect of Q cells. Future research should focus on understanding the mechanisms influencing Q cells and developing targeted therapies to improve patient outcomes.
Keywords: GBM; PBZ; SMR; imaging; microenvironment; physics.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Differential gene expression in peritumoral brain zone of glioblastoma: role of SERPINA3 in promoting invasion, stemness and radioresistance of glioma cells and association with poor patient prognosis and recurrence.J Neurooncol. 2021 Mar;152(1):55-65. doi: 10.1007/s11060-020-03685-4. Epub 2021 Jan 3. J Neurooncol. 2021. PMID: 33389566
-
Morphological differentiation of peritumoral brain zone microglia.PLoS One. 2024 Mar 7;19(3):e0297576. doi: 10.1371/journal.pone.0297576. eCollection 2024. PLoS One. 2024. PMID: 38451958 Free PMC article.
-
Intratumoral heterogeneity in glioblastoma: don't forget the peritumoral brain zone.Neuro Oncol. 2015 Oct;17(10):1322-32. doi: 10.1093/neuonc/nov119. Epub 2015 Jul 22. Neuro Oncol. 2015. PMID: 26203067 Free PMC article. Review.
-
Advanced Imaging Techniques for Differentiating Pseudoprogression and Tumor Recurrence After Immunotherapy for Glioblastoma.Front Immunol. 2021 Nov 25;12:790674. doi: 10.3389/fimmu.2021.790674. eCollection 2021. Front Immunol. 2021. PMID: 34899760 Free PMC article. Review.
-
Magnetic resonance imaging-guided intracranial resection of glioblastoma tumors in patient-derived orthotopic xenografts leads to clinically relevant tumor recurrence.BMC Cancer. 2024 Jan 2;24(1):3. doi: 10.1186/s12885-023-11774-6. BMC Cancer. 2024. PMID: 38166949 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

