Long-term comparison of maxillary protraction with hybrid hyrax-facemask vs. hybrid hyrax-mentoplate protocols using Alt-RAMEC: a 5-year randomized controlled trial
- PMID: 40105065
- PMCID: PMC11920792
- DOI: 10.1093/ejo/cjaf011
Long-term comparison of maxillary protraction with hybrid hyrax-facemask vs. hybrid hyrax-mentoplate protocols using Alt-RAMEC: a 5-year randomized controlled trial
Abstract
Background: The study aimed to compare the short- and long-term effectiveness of hybrid Hyrax (HH) -Facemask (FM) and HH-mentoplate (MP) treatment protocols for maxillary protraction using Alt-RAMEC.
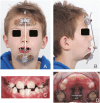
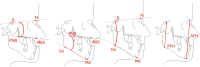
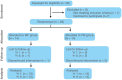
Methods: A single-center 2-arm parallel randomized controlled trial. Participants: 28 skeletal class III patients (female: 14, male: 14; average age: 9.7 ± 1.3 years;) were included. Interventions: Two treatment groups where protraction therapy was combined with Alt-RAMEC. Group 1: Facemask group (Hybrid Hyrax + Facemask) and Group 2: Mentoplate group (Hybrid Hyrax + Mentoplate). Objective: To compare skeletal and dental changes between groups using low dose computed tomography (CT) scan from which virtual lateral cephalograms were generated. Outcome: Outcomes include changes in Wits appraisal (primary outcome), and cephalometric analysis of skeletal and dental changes (secondary outcomes) at 1 year and 5 years after treatment initiation. Randomization: 28 patients were allocated to either treatment-protocols using sequentially numbered opaque, sealed envelopes. The randomization sequence was generated with a 1:1 allocation ratio. Blinding: Due to the nature of the trial, the operator and children could not be blinded to the treatment allocation. However, blinding was used when assessing the outcomes.
Results: Follow-up: one patient was lost at the one-year follow-up and an additional three patients were lost at the 5-year follow-up. Outcomes: Both treatment protocols effectively improved intermaxillary relationship. Wits measurements showed improvements of 4.42 mm (FM) and 2.86 mm (MP) at T1, decreasing slightly to 3.33 mm (FM) and 1.50 mm (MP) at T2. While vertical control and incisor inclination were comparable between groups long-term, short-term differences were noted in upper and lower incisor inclination. Results remained equally stable after five years (T2). Harms: minor harms were encountered with the anchor hooks (fracture or mucosal irritation), however none led to treatment cessation.
Conclusions: Early class III treatment with HH + MP provided similar outcomes and stability to that of HH + FM suggesting that the choice between FM and MP should be based on individual patient factors rather than presumed mechanical advantages.
Trial registration: Clinical Trials ID: NCT02711111.
Keywords: bone anchored; facemask; maxillofacial protraction; orthodontics; orthopedic therapy; skeletal class III malocclusion.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Orthodontic Society.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Woon SC, Thiruvenkatachari B.. Early orthodontic treatment for Class III malocclusion: a systematic review and meta-analysis. Am J Orthod Dentofacial Orthop 2017;151:28–52. https://doi.org/10.1016/j.ajodo.2016.07.017 - DOI - PubMed
-
- De Toffol L, Pavoni C, Baccetti T, et al.Orthopedic treatment outcomes in Class III malocclusion. Angle Orthod 2008;78:561–73. https://doi.org/10.2319/030207-108.1 - DOI - PubMed
-
- Rongo R, D’Antò V, Bucci R, et al.Skeletal and dental effects of Class III orthopaedic treatment: a systematic review and meta-analysis. J Oral Rehabil 2017;44:545–62. https://doi.org/10.1111/joor.12495 - DOI - PubMed
-
- Ngan PW, Hagg U, Yiu C, et al.Treatment response and long-term dentofacial adaptations to maxillary expansion and protraction. Semin Orthod 1997;3:255–64. https://doi.org/10.1016/s1073-8746(97)80058-8 - DOI - PubMed
-
- Cevidanes L, Baccetti T, Franchi L, et al.Comparison of two protocols for maxillary protraction: bone anchors versus face mask with rapid maxillary expansion. Angle Orthod 2010;80:799–806. https://doi.org/10.2319/111709-651.1 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

