Genetic dissection of MutL complexes in Arabidopsis meiosis
- PMID: 40105242
- PMCID: PMC11920794
- DOI: 10.1093/nar/gkaf187
Genetic dissection of MutL complexes in Arabidopsis meiosis
Abstract
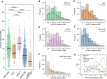
During meiosis, homologous chromosomes exchange genetic material through crossing over. The main crossover pathway relies on ZMM proteins, including ZIP4 and HEI10, and is typically resolved by the MLH1/MLH3 heterodimer, MutLγ. Our analysis shows that while MUS81 may partially compensate for MutLγ loss, its role remains uncertain. However, our multiple mutant analysis shows that MUS81 is unlikely to be the sole resolvase of ZMM-protected recombination intermediates when MutLγ is absent. Comparing genome-wide crossover maps of mlh1 mutants with ZMM-deficient mutants and lines with varying HEI10 levels reveals that crossover interference persists in mlh1 but is weakened. The significant crossover reduction in mlh1 also increases aneuploidy in offspring. The loss of MutLγ can be suppressed by eliminating the FANCM helicase. Combined with the lower-than-expected chiasma frequency, this suggests that in MutLγ absence, some ZMM-protected intermediates are ultimately resolved by DNA helicases and/or their complexes with Top3α. Elevated MLH1 or MLH3 expression moderately increases crossover frequency, while their misregulation drastically reduces crossover numbers and plant fertility, highlighting the importance for tight control of MLH1/MLH3 levels. By contrast, PMS1, a component of the MutLα endonuclease, appears uninvolved in crossing over. Together, these findings demonstrate the unique role of MutLγ in ZMM-dependent crossover regulation.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
None declared.
Figures







References
MeSH terms
Substances
Grants and funding
- 2020/39/I/NZ2/02464/National Science Center, Poland (NCN)
- PID2020-118038GB-I00/AEI/10.13039/501100011033/Ministry of Science and Innovation of Spain
- TED2021-131852B-I00/AEI/10.13039/501100011033/Unión/European Union
- Initiative of Excellence-Research University at Adam Mickiewicz University, Poznan, Poland
LinkOut - more resources
Full Text Sources
Research Materials

