Photocatalyzed Epimerization of Quaternary Stereocenters
- PMID: 40105282
- PMCID: PMC11969547
- DOI: 10.1021/jacs.4c16769
Photocatalyzed Epimerization of Quaternary Stereocenters
Abstract
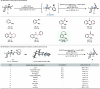
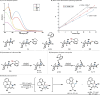
Quaternary stereocenters play a crucial role in shaping both the molecular topology of small molecules and the outcome of stereoselective transformations. While considerable progress has been achieved in constructing highly substituted carbon centers with varied substitution patterns, the stereoselective synthesis of quaternary carbon centers remains a significant challenge. Here we report a protocol for the precise manipulation of quaternary stereocenters through epimerization. The critical design element of our ketone α-epimerization process was developing a photoactive imine, which circumvents the numerous deleterious pathways of carbonyl photochemistry. Excitation of this imine with visible light in the presence of a photocatalyst enables reversible C-C bond cleavage and reformation to vary the stereochemistry of the quaternary center. This approach allows us to override intrinsic stereochemical outcomes of C-C bond construction, therefore providing novel tactics for retrosynthetic planning. The broad utility of this protocol was demonstrated by the topological alteration of various classes of carbocyclic scaffolds bearing diverse functional groups.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Xin Z.; Wang H.; He H.; Gao S. Recent advances in the total synthesis of natural products bearing the contiguous all-carbon quaternary stereocenters. Tetrahedron Lett. 2021, 71, 153029.10.1016/j.tetlet.2021.153029. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

