Neoadjuvant Chemoradiotherapy vs Chemoimmunotherapy for Esophageal Squamous Cell Carcinoma
- PMID: 40105813
- PMCID: PMC11923775
- DOI: 10.1001/jamasurg.2025.0220
Neoadjuvant Chemoradiotherapy vs Chemoimmunotherapy for Esophageal Squamous Cell Carcinoma
Abstract
Importance: The association of neoadjuvant chemoimmunotherapy (NCIT) vs chemoradiotherapy (NCRT) with tumor downstaging and survival in locally advanced esophageal squamous cell carcinoma (ESCC) remains unclear because of limited evidence.
Objective: To compare the associations of NCIT and NCRT with tumor regression and long-term survival in patients with locally advanced ESCC.
Design, setting, and participants: In this comparative effectiveness research study, from January 2016 to March 2023, patients with locally advanced ESCC who underwent esophagectomy following NCRT or NCIT were identified from a prospective database of 8 high-volume esophageal surgery centers in China. Follow-up began on the date of surgery and continued until the last recorded contact or March 2024, whichever occurred first. Data were analyzed between April and September 2024.
Main outcomes and measures: The primary end points were 2-year overall survival (OS) and disease-free survival (DFS). Secondary end points included major pathologic response (MPR) and pathologic complete response (pCR). Cox proportional hazard regression analysis was used to investigate the risk factors for OS and DFS.
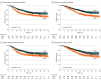
Results: The study included 1428 patients (median [IQR] age, 63 [57-68] years; 1184 men [82.9%]), with 704 patients in the NCRT group and 724 patients in the NCIT group. After propensity score matching, there were 532 patients in each group. The 2-year OS (81.3% vs 71.3%; hazard ratio, 1.57; 95% CI, 1.26-1.96; P < .001) and DFS (73.9% vs 63.4%; hazard ratio, 1.37; 95% CI, 1.11-1.69; P < .001) rates were significantly higher in NCIT group than in the NCRT group. The NCRT group had a higher MPR rate than that of the NCIT group (71.8% vs 61.5%), whereas the pCR rates were similar (25.9% vs 22.9%). Multivariable Cox analysis demonstrated that NCIT and MPR were independently associated with both OS and DFS. The NCIT group exhibited a lower overall recurrence rate (126 patients [23.7%] vs 190 patients [35.7%]) and distant metastasis rate (72 patients [13.5%] vs 133 patients [25.0%]), although locoregional metastasis rates were similar (98 patients [18.4%] vs 111 patients [20.9%]). Better OS and DFS were obtained for the NCIT group than for the NCRT group, regardless of whether adjuvant immunotherapy was given.
Conclusions and relevance: Compared with NCRT, patients with locally advanced ESCC receiving NCIT had better 2-year OS and DFS. The decrease in distant metastasis may be the main reason, but further prospective randomized clinical trials are needed to verify this finding.
Conflict of interest statement
Figures

Comment on
-
Will Care for Esophageal Squamous Cell Carcinoma Change?JAMA Surg. 2025 May 1;160(5):575. doi: 10.1001/jamasurg.2025.0232. JAMA Surg. 2025. PMID: 40105818 No abstract available.
References
-
- Yang H, Liu H, Chen Y, et al. ; AME Thoracic Surgery Collaborative Group . Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796-2803. doi:10.1200/JCO.2018.79.1483 - DOI - PMC - PubMed
-
- Kato K, Machida R, Ito Y, et al. ; JCOG1109 investigators . Doublet chemotherapy, triplet chemotherapy, or doublet chemotherapy combined with radiotherapy as neoadjuvant treatment for locally advanced oesophageal cancer (JCOG1109 NExT): a randomised, controlled, open-label, phase 3 trial. Lancet. 2024;404(10447):55-66. doi:10.1016/S0140-6736(24)00745-1 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

