Challenges in managing iTTP: insights into ADAMTS13 inhibitor boosting during caplacizumab therapy
- PMID: 40105947
- PMCID: PMC12031809
- DOI: 10.1007/s00277-025-06318-w
Challenges in managing iTTP: insights into ADAMTS13 inhibitor boosting during caplacizumab therapy
Abstract
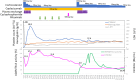
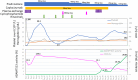
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a rare but life-threatening disorder characterized by severe thrombocytopenia, hemolytic anemia, and end-organ ischemic damage. The introduction of caplacizumab, an anti-von Willebrand factor A1 nanobody, has revolutionized the treatment of patients with iTTP by preventing fatal thrombotic events and shortening the time to platelet normalization. Despite its benefits, caplacizumab does not address the challenge of anti-ADAMTS13 autoantibody production, posing a risk of ADAMTS13 inhibitor boosting and delayed recovery of ADAMTS13 activity. Here, we highlight three challenging cases from the Japanese TTP registry involving patients with iTTP who experienced severe ADAMTS13 inhibitor boosting. This delayed the recovery of ADAMTS13, and extended administration of caplacizumab while requiring additional therapeutic plasma exchange (TPE) and immunosuppressive therapy. All patients demonstrated delayed recovery of ADAMTS13 activity despite initial clinical improvement. Prolonged use of caplacizumab masked the persistence of ADAMTS13 inhibitors, emphasizing the need for close monitoring and timely interventions. Although recent proposals for TPE-free regimens show promise, our findings underscore that TPE remains essential for removing residual autoantibodies and preventing disease exacerbation in certain patients. Stratifying patients based on initial ADAMTS13 inhibitor titers and optimizing immunosuppressive strategies may help identify those at risk of severe inhibitor boosting. Further research is required to refine treatment protocols and ensure the safe withdrawal of caplacizumab while achieving sustained recovery of ADAMTS13 activity.
Keywords: ADAMTS13; Autoantibodies; Caplacizumab; Therapeutic plasma exchange; Thrombotic thrombocytopenic purpura.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: K. S. received speaker fees from Sanofi and participated on the advisory boards for Takeda. Y. U. received speaker fees from Sanofi. M.M. provided consultancy services for Takeda, Alexion Pharma, and Sanofi; received speaker fees from Takeda, Alexion Pharma, Asahi Kasei Pharma, and Sanofi; and received research funding from Alexion Pharma, Chugai Pharmaceutical, Asahi Kasei Pharma, and Sanofi. Consent to publish: Not applicable.
Figures

Similar articles
-
Immune Thrombotic Thrombocytopenic Purpura: A Review.JAMA. 2025 Aug 12;334(6):517-529. doi: 10.1001/jama.2025.3807. JAMA. 2025. PMID: 40388146 Review.
-
Persistent ADAMTS13 inhibitor delays recovery of ADAMTS13 activity in caplacizumab-treated Japanese patients with iTTP.Blood Adv. 2024 May 14;8(9):2151-2159. doi: 10.1182/bloodadvances.2023012451. Blood Adv. 2024. PMID: 38386976 Free PMC article.
-
Refining the standard of care in immune thrombotic thrombocytopenic purpura.Clin Adv Hematol Oncol. 2024 Oct;22(8):381-391. Clin Adv Hematol Oncol. 2024. PMID: 39356816 Review.
-
Management of immune thrombotic thrombocytopenic purpura without therapeutic plasma exchange.Blood. 2024 Oct 3;144(14):1486-1495. doi: 10.1182/blood.2023023780. Blood. 2024. PMID: 38838300
-
Caplacizumab: a change in the paradigm of thrombotic thrombocytopenic purpura treatment.Expert Opin Biol Ther. 2019 Nov;19(11):1127-1134. doi: 10.1080/14712598.2019.1650908. Epub 2019 Aug 5. Expert Opin Biol Ther. 2019. PMID: 31359806 Review.
References
-
- Kremer Hovinga JA, Coppo P, Lammle B, Moake JL, Miyata T, Vanhoorelbeke K (2017) Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers 3:17020. 10.1038/nrdp.2017.20 - PubMed
-
- Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spasoff RA (1991) Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian apheresis study group. N Engl J Med 325(6):393–397. 10.1056/NEJM199108083250604 - PubMed
-
- Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knobl P, Wu H, Artoni A, Westwood JP, Mansouri Taleghani M, Jilma B, Callewaert F, Ulrichts H, Duby C, Tersago D, Investigators T (2016) Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med 374(6):511–522. 10.1056/NEJMoa1505533 - PubMed
-
- Scully M, Cataland SR, Peyvandi F, Coppo P, Knobl P, Kremer Hovinga JA, Metjian A, de la Rubia J, Pavenski K, Callewaert F, Biswas D, De Winter H, Zeldin RK, Investigators H (2019) Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med 380(4):335–346. 10.1056/NEJMoa1806311 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

