A novel peptide targeting CCR7 inhibits tumor cell lymph node metastasis
- PMID: 40105966
- PMCID: PMC11923353
- DOI: 10.1007/s00262-025-03995-4
A novel peptide targeting CCR7 inhibits tumor cell lymph node metastasis
Abstract
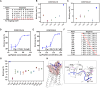
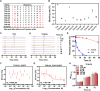
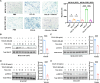
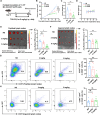
Lymph nodes are the most common metastasis sites for tumor cells, which are intimately linked to patient prognosis. It has been reported that cancer cells can upregulate CC Chemokine Receptor 7 (CCR7) expression and hijack its normal functions, enabling them to migrate along the gradient of CCL19 and CCL21 toward the lymph nodes and colonies as the initial stage of distant metastasis. In tumor patients, the metastatic tumor in the lymph nodes exhibited higher expression of CCR7, as well as inhibitory immune checkpoints PD-1, LAG-3, and TIM-3 compared to the primary tumors with the analysis of TCGA and GEO databases. Also, in mouse tumor model, tumor cells with elevated CCR7 expression were more susceptible to develop popliteal lymph node metastasis. Subsequently, we successfully identified a CCR7 binding peptide TC6 by phage display biopanning, which specifically blocks the interaction of CCR7/CCL19 and CCR7/CCL21. Further, the D-amino acids were introduced to substitute the N- and C-terminus of TC6 peptide to obtain the proteolysis-resistant TC6-D3 peptide, which decreased tumor cell migration in vitro via ERK1/2 pathway and inhibited tumor growth and lymph nodes metastasis in vivo, as well as effectively restored T cells cytotoxicity in both primary tumors and lymph nodes. In conclusion, CCR7 promoted tumor cell metastasis to lymph node and inhibited the anti-tumor immune responses in lymph nodes. Specific blockade of the CCR7 pathway with TC6-D3 peptide can significantly reduce lymph node tumor burden, promoting CD8+ T cell infiltration in primary tumors, meanwhile, enhancing anti-tumor immune responses in lymph nodes.
Keywords: Blockade; CCR7; Immune responses; Lymph node metastasis; Peptide.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures


Similar articles
-
The expression of chemokines CCL19, CCL21 and their receptor CCR7 in oral squamous cell carcinoma and its relevance to cervical lymph node metastasis.Tumour Biol. 2013 Feb;34(1):65-70. doi: 10.1007/s13277-012-0511-3. Epub 2012 Sep 14. Tumour Biol. 2013. PMID: 22976543
-
Expression of chemokine receptor CCR7 is associated with cervical lymph node metastasis of oral squamous cell carcinoma.Oral Oncol. 2009 Jun;45(6):480-5. doi: 10.1016/j.oraloncology.2008.06.005. Epub 2008 Aug 26. Oral Oncol. 2009. PMID: 18752985
-
Fluorescently Tagged CCL19 and CCL21 to Monitor CCR7 and ACKR4 Functions.Int J Mol Sci. 2018 Dec 4;19(12):3876. doi: 10.3390/ijms19123876. Int J Mol Sci. 2018. PMID: 30518137 Free PMC article.
-
Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes.J Leukoc Biol. 2016 Jun;99(6):869-82. doi: 10.1189/jlb.2MR0815-380R. Epub 2016 Jan 4. J Leukoc Biol. 2016. PMID: 26729814 Review.
-
[Progress in targeting therapy of cancer metastasis by CCL21/CCR7 axis].Sheng Wu Gong Cheng Xue Bao. 2020 Dec 25;36(12):2741-2754. doi: 10.13345/j.cjb.200174. Sheng Wu Gong Cheng Xue Bao. 2020. PMID: 33398969 Review. Chinese.
Cited by
-
A Beautiful Bind: Phage Display and the Search for Cell-Selective Peptides.Viruses. 2025 Jul 12;17(7):975. doi: 10.3390/v17070975. Viruses. 2025. PMID: 40733592 Free PMC article. Review.
References
-
- Al-Sukhni E, Attwood K, Gabriel EM, LeVea CM, Kanehira K, Nurkin SJ (2017) Lymphovascular and perineural invasion are associated with poor prognostic features and outcomes in colorectal cancer: a retrospective cohort study. Int J Surg 37:42–49. 10.1016/j.ijsu.2016.08.528 - PubMed
MeSH terms
Substances
Grants and funding
- 24qnpy187/The Fundamental Research Funds for the Central Universities, Sun Yat-sen University
- 24xkjc021/The Fundamental Research Funds for the Central Universities, Sun Yat-sen University
- KQTD20190929173853397/Shenzhen Science and Technology Program
- 2019ZT08Y464/The "Pearl River Talent Plan" Innovation and Entrepreneurship Team Project of Guangdong Province
- 2022B1515120085/The Guangdong Basic and Applied Basic Research Foundation
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

