Application of the anti-IgLON5 disease composite score to assess severity, clinical course, and mortality in a French cohort
- PMID: 40106089
- PMCID: PMC11922985
- DOI: 10.1007/s00415-025-13001-7
Application of the anti-IgLON5 disease composite score to assess severity, clinical course, and mortality in a French cohort
Abstract
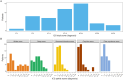
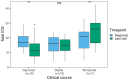
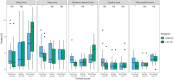
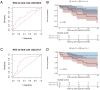
Anti-IgLON5 disease presents with diverse symptoms, whose severity can be measured by the anti-IgLON5 disease composite score (ICS). This study applied the ICS to a retrospective anti-IgLON5 disease cohort (n = 52; median age 72 years, 63% male) diagnosed in the French Reference Center on Autoimmune Encephalitis (2016-2024), aiming to describe severity and clinical course, and to assess its potential to predict mortality. At diagnosis, the ICS distribution (median 18) aligned with previous publications and correlated with the time to diagnosis (median 19 months); all patients had symptoms in ≥ 2 ICS domains: bulbar (88%), sleep (84%), movement disorders (90%), cognition (64%), and/or other (78%). Of 46 patients with follow-up data, 7 (16%) died shortly after diagnosis; for the others, changes in the ICS mirrored the clinical course: at last visit, it decreased in improving patients (16/46, 35%; median 12 vs 17; p = 0.004), increased in worsening patients (11/39, 24%; median 26 vs 21; p = 0.006) and did not change significantly in stable patients (12/46, 26%; median 16 vs 15; p = 0.222). In the ROC analyses, 2-year mortality was predicted by the total ICS at diagnosis (AUC 69.51, 95% CI [50.19; 88.83]; optimal cut-off > 20, sensitivity 59%, specificity 77%), and by the bulbar score at diagnosis (AUC 74.68, 95% CI [56.17, 93.19]; optimal cut-off > 3, sensitivity 83%, specificity 62%). The ICS is a reproducible tool for assessing anti-IgLON5 disease severity and clinical course. Higher total and bulbar ICS at diagnosis are associated with increased mortality risk, underscoring the need for early and intensive management of bulbar dysfunction.
Keywords: Anti-IgLON5 antibodies; Anti-IgLON5 disease; Autoimmune encephalitis; Neuronal surface antigen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: Macarena Villagrán-García is supported by a research grant from Fundación Alfonso Martín Escudero (Spain). Antonio Farina received a grant to perform research abroad by the European Academy of Neurology in 2022. The other authors report no competing interests. Ethical standards statement: This study was approved by the institutional review board of the Hospices Civils of Lyon (N 23-5083). Written information was sent to all patients and none of them explicitly opposed his/her participation in the study.
Figures
References
-
- Sabater L, Gaig C, Gelpi E, Bataller L, Lewerenz J, Torres-Vega E, Contreras A, Giometto B, Compta Y, Embid C et al (2014) A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol 13:575–586 - PMC - PubMed
-
- Landa J, Gaig C, Plagumà J, Saiz A, Antonell A, Sanchez-Valle R, Dalmau J, Graus F, Sabater L (2020) Effects of IgLON5 antibodies on neuronal cytoskeleton: a link between autoimmunity and neurodegeneration. Ann Neurol 88:1023–1027 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

