Real-World Use, Effectiveness, and Safety of Intravenous Fosfomycin: The FORTRESS Study
- PMID: 40106180
- PMCID: PMC11993532
- DOI: 10.1007/s40121-025-01125-2
Real-World Use, Effectiveness, and Safety of Intravenous Fosfomycin: The FORTRESS Study
Abstract
Introduction: Intravenous fosfomycin (FOS) is a broad-spectrum antibiotic primarily used in combination therapy to treat severe infections caused by both Gram-positive (GP) and Gram-negative (GN) pathogens, including multi-drug resistant (MDR) bacteria. The aim of this study, the largest to date, was to evaluate the effectiveness, safety, usage patterns, and patient characteristics of FOS in a real-world setting.
Methods: Interim analysis of an ongoing, prospective, non-interventional, multicentre study in five European countries, involving centres in Germany, Italy, the United Kingdom, Greece, and Austria.
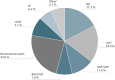
Results: A total of 716 patients were enrolled between January 2017 and November 2023 (mean age: 62.8 years, APACHE II: 18.3, SOFA: 6.7). Main indications for FOS were bacteraemia/sepsis (23.6%), complicated urinary tract infections (18.0%), and bone and joint infections (17.4%). Other indications included hospital-acquired/ventilator-associated pneumonia (11.0%), complicated skin and soft tissue infections (9.1%), bacterial meningitis/central nervous system (CNS) infections (7.8%), and infective endocarditis (6.4%). Most common pathogens identified were Staphylococcus aureus (31.4%, including methicillin-resistant S. aureus), Klebsiella spp. (including K. pneumoniae) (17.2%), Escherichia coli (14.2%), coagulase-negative staphylococci (12.9%), other Enterobacterales (10.9%), and Pseudomonas aeruginosa (8.4%). In 34.6% of patients, an MDR pathogen was involved. Carbapenem resistance (CR) was high in Klebsiella spp. infections (59/123, 48.0%). In most patients, FOS was used in combination therapy (90.2%). The median dose was 15 g/day. Overall, clinical success and clinical response were favourable with 75.3% and 83.4% at the end of FOS treatment. Clinical success rates in infections caused by MDR or CR pathogens were 78.0% and 81.8%, respectively. Microbiological cure was achieved in 82.4% of all patients. Electrolyte imbalances were the most frequently observed adverse drug reactions, while gastrointestinal disorders were rare.
Conclusion: The results from this study suggest that FOS is a safe and effective option as combination partner in the treatment of patients with severe infections caused by both GP and GN pathogens, including deep-seated infections and/or involvement of MDR bacteria.
Trial registration: ClinicalTrials.gov identifier, NCT02979951.
Keywords: Klebsiella pneumoniae; Pseudomonas aeruginosa; Staphylococcus aureus; Bacteraemia; Carbapenem-resistant Enterobacterales; Fosfomycin; Infective endocarditis; MDR; Observational study; Sepsis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Klaus-Friedrich Bodmann has received honoraria from Shionogi, Advanz Pharma, Gilead, Roche, Eli Lilly, and InfectoPharm. Stefan Hagel has received honoraria from Pfizer, MSD, InfectoPharm, Philips, Advanz Pharma, Beckman Coulter, Shionogi, Thermo Fisher, and Tillots. He also participated in advisory boards for Advanz Pharma, Shionogi, and Pfizer. Alessandra Oliva has received honoraria from Advanz Pharma, MSD, InfectoPharm, and Pfizer. She also participated in advisory boards for Advanz Pharma. Stefan Kluge received research support from Cytosorbents and Daiichi Sankyo. He also received lecture fees from ADVITOS, Biotest, CSL Behring, Daiichi Sankyo, Fresenius Medical Care, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer, Shionogi, and Zoll. He received consultant fees from ADVITOS, AstraZeneca, Fresenius, Gilead, MSD, and Pfizer. Alessandra Mularoni has received honoraria from Pfizer, Gilead, and Takeda and participated in advisory boards for Takeda and MSD. Marco Falcone received honoraria from Pfizer, Menarini, Gilead, GSK, and Thermo Fisher. Mathias W. Pletz has received honoraria from Pfizer, MSD, Sanofi, Janssen, GSK, AstraZeneca, InfectoPharm, and Shionogi. Jan T. Kielstein received speaker fees from Fresenius Medical Care, BAXTER, and DiaMed. Carlo Tascini has received honoraria from Angelini, InfectoPharm, Menarini, Correvio, Merck, Viatris, Pfizer, Thermo Fisher, Diasorin, and Biomérieux. He also participated in advisory boards for Pfizer, Viatris, and Thermo Fisher. Claudia Spies participated in advisory boards for Takeda and Lynx Health Science. Alessandra Bandera has received honoraria from AstraZeneca, Biomérieux, Qiagen, Janssen-Cilag, and Nordic Pharma. She has also participated in advisory boards for Viiv Healthcare, Sobi, Gilead, and Angelini Pharma. Matthias G. Vossen received consulting fees and honoraria for educational talks from Astro Pharma. Abhijit M. Bal is an investigator in the UKAR trial and PROVE trial. Thomas Borrmann and Christian Mayer are employees of InfectoPharm, Heppenheim, Germany. Valentina Galfo, Simone Lindau, Nadja Käding, Michael Zoller, Sebastian Kintrup, Dirk Schädler, Francesco G. De Rosa, Szilvia Radnoti, Roberto Luzzati, Sam Allen, Loredana Sarmati, Antonio Cascio, Nikolaos Kapravelos, Chinari P. K. Subudhi, George Dimopoulos, Mario Venditti, and Claudio M. Mastroianni have nothing to declare. Ethical Approval: The study was conducted in accordance with the Helsinki Declaration of 1964 (and its amendments) and was approved by all ethics committees or other authorities according to national/local requirements (Supplementary Table S13). Written informed consent was obtained from each patient or the patient’s legally acceptable representative before any study-specific activity was performed. In Austria, an informed consent waiver was approved for patients who were incapacitated.
Figures
References
-
- Oliva A, Volpicelli L, Di Bari S, Curtolo A, Borrazzo C, Cogliati Dezza F, et al. Effect of ceftazidime/avibactam plus fosfomycin combination on 30 day mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae: results from a multicentre retrospective study. JAC Antimicrob Resist. 2022;4(6):dlac121. - PMC - PubMed
-
- Tseng TC, Chuang YC, Yang JL, Lin CY, Huang SH, Wang JT, et al. The combination of daptomycin with fosfomycin is more effective than daptomycin alone in reducing mortality of vancomycin-resistant enterococcal bloodstream infections: a retrospective, comparative cohort study. Infect Dis Ther. 2023;12(2):589–606. - PMC - PubMed
-
- Grabein B, Graninger W, Rodríguez Baño J, Dinh A, Liesenfeld DB. Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature. Clin Microbiol Infect. 2017;23(6):363–72. - PubMed
-
- Khawcharoenporn T, Chuncharunee A, Maluangnon C, Taweesakulvashra T, Tiamsak P. Active monotherapy and combination therapy for extensively drug-resistant Pseudomonas aeruginosa pneumonia. Int J Antimicrob Agents. 2018;52(6):828–34. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

