Activin a regulates vascular formation and stabilization in direct coculture of dental pulp stem cells and endothelial cells
- PMID: 40106315
- PMCID: PMC12160993
- DOI: 10.1111/iej.14226
Activin a regulates vascular formation and stabilization in direct coculture of dental pulp stem cells and endothelial cells
Abstract
Aim: Establishing functional circulation on time is crucial to dental pulp tissue regeneration. Mesenchymal stem cells (MSCs) could act as mural cells to stabilize newly formed blood vessels, accelerating anastomosis. Our preliminary study found that direct coculture of dental pulp stem cells (DPSCs) and human umbilical vein endothelial cells (HUVECs) significantly enhanced Activin A secretion. This study aimed to disclose the dynamic patterns of Activin A expression and its regulation on vascular formation and stabilization.
Methodology: DPSCs and HUVECs were cocultured directly at a ratio of 1:1 for 3 and 6 days. Activin A and Follistatin expression were evaluated by qRT-PCR and ELISA. HUVECs were exposed to 100 ng/mL Activin A or the conditioned medium (CM) generated from DPSC monoculture and DPSC-HUVEC coculture, respectively. HUVEC proliferation, migration, tube formation and angiogenic sprouting were assessed. In parallel, membrane-bound vascular endothelial growth factor receptors (mVEGFR1 and mVEGFR2) and soluble VEGFR1 (sVEGFR1) were analysed at days 3 and 6.
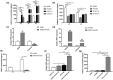
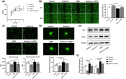
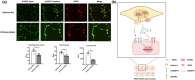
Results: Activin A expression and secretion were elevated time-dependently during DPSC-HUVEC coculture. Follistatin expression decreased in DPSC-HUVEC coculture while the ratio of Activin A/Follinstain increased significantly. Activin A treatment did not promote DPSC towards smooth muscle cell (SMC)-specific differentiation, while Activin A and DPSC+HUVEC-CM suppressed HUVEC proliferation, migration, tube formation and sprouting. Activin A and DPSC+HUVEC-CM treatment markedly increased mVEGFR1 expression and sVEGFR1 secretion, suppressing HUVEC vascular formation. Activin A IgG partially reversed the effects of DPSC+HUVEC-CM on HUVECs by decreasing VEGFR1 expression and increasing vessel formation. Activin A pretreatment downregulated VEGF-triggered VEGFR2 phosphorylation of HUVECs. INHBA knockdown DPSCs disrupted the stabilization of the preformed HUVEC vascular tube network.
Conclusion: DPSC-HUVEC direct coculture upregulates Activin A secretion, interrupting VEGF receptors' balance in HUVECs to suppress HUVEC angiogenic sprouting and enhance vascular stabilization. These findings provide novel insights into the paracrine interactions on vascular stabilization of DPSC-HUVEC direct coculture.
Keywords: Activin A; angiogenesis; dental pulp stem cells; endothelial cells; vascular endothelial growth factor receptors; vascular stabilization.
© 2025 The Author(s). International Endodontic Journal published by John Wiley & Sons Ltd on behalf of British Endodontic Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Direct contact with endothelial cells drives dental pulp stem cells toward smooth muscle cells differentiation via TGF-β1 secretion.Int Endod J. 2023 Sep;56(9):1092-1107. doi: 10.1111/iej.13943. Epub 2023 Jun 20. Int Endod J. 2023. PMID: 37294792
-
DPSCs treated by TGF-β1 regulate angiogenic sprouting of three-dimensionally co-cultured HUVECs and DPSCs through VEGF-Ang-Tie2 signaling.Stem Cell Res Ther. 2021 May 10;12(1):281. doi: 10.1186/s13287-021-02349-y. Stem Cell Res Ther. 2021. PMID: 33971955 Free PMC article.
-
ATP1A1-Driven Intercellular Contact Between Dental Pulp Stem Cell and Endothelial Cell Enhances Vasculogenic Activity.Int Dent J. 2025 Jul 7;75(5):100870. doi: 10.1016/j.identj.2025.100870. Online ahead of print. Int Dent J. 2025. PMID: 40628202 Free PMC article.
-
Specific microRNAs Regulate Dental Pulp Stem Cell Behavior.J Endod. 2022 Jun;48(6):688-698. doi: 10.1016/j.joen.2022.02.012. Epub 2022 Mar 7. J Endod. 2022. PMID: 35271859
-
Angiogenic and neurogenic potential of dental-derived stem cells for functional pulp regeneration: A narrative review.Int Endod J. 2025 Mar;58(3):391-410. doi: 10.1111/iej.14180. Epub 2024 Dec 11. Int Endod J. 2025. PMID: 39660369 Review.
References
-
- Bloise, E. , Ciarmela, P. , Dela Cruz, C. , Luisi, S. , Petraglia, F. & Reis, F.M. (2019) Activin a in mammalian physiology. Physiological Reviews, 99(1), 739–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

