Cost-effectiveness of broadly neutralizing antibodies for HIV prophylaxis for infants born in settings with high HIV burdens
- PMID: 40106399
- PMCID: PMC11922280
- DOI: 10.1371/journal.pone.0318940
Cost-effectiveness of broadly neutralizing antibodies for HIV prophylaxis for infants born in settings with high HIV burdens
Abstract
Background: Approximately 130 000 infants acquire HIV annually despite global maternal antiretroviral therapy scale-up. We evaluated the potential clinical impact and cost-effectiveness of offering long-acting, anti-HIV broadly neutralizing antibody (bNAb) prophylaxis to infants in three distinct settings.
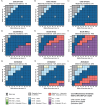
Methods: We simulated infants in Côte d'Ivoire, South Africa, and Zimbabwe using the Cost-Effectiveness of Preventing AIDS Complications-Pediatric (CEPAC-P) model. We modeled strategies offering a three-bNAb combination in addition to WHO-recommended standard-of-care oral prophylaxis to infants: a) with known, WHO-defined high-risk HIV exposure at birth (HR-HIVE); b) with known HIV exposure at birth (HIVE); or c) with or without known HIV exposure (ALL). Modeled infants received 1-dose, 2-doses, or Extended (every 3 months through 18 months) bNAb dosing. Base case model inputs included 70% bNAb efficacy (sensitivity analysis range: 10-100%), 3-month efficacy duration/dosing interval (1-6 months), and $20/dose cost ($5-$100/dose). Outcomes included pediatric HIV infections, life expectancy, lifetime HIV-related costs, and incremental cost-effectiveness ratios (ICERs, in US$/year-of-life-saved [YLS], assuming a ≤ 50% GDP per capita cost-effectiveness threshold).
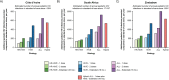
Findings: The base case model projects that bNAb strategies targeting HIVE and ALL infants would prevent 7-26% and 10-42% additional pediatric HIV infections, respectively, compared to standard-of-care alone, ranging by dosing approach. HIVE-Extended would be cost-effective (cost-saving compared to standard-of-care) in Côte d'Ivoire and Zimbabwe; ALL-Extended would be cost-effective in South Africa (ICER: $882/YLS). BNAb strategies targeting HR-HIVE infants would result in greater lifetime costs and smaller life expectancy gains than HIVE-Extended. Throughout most bNAb efficacies and costs evaluated in sensitivity analyses, targeting HIVE infants would be cost-effective in Côte d'Ivoire and Zimbabwe, and targeting ALL infants would be cost-effective in South Africa.
Interpretation: Adding long-acting bNAbs to current standard-of-care prophylaxis would be cost-effective, assuming plausible efficacies and costs. The cost-effective target population would vary by setting, largely driven by maternal antenatal HIV prevalence and postpartum incidence.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
SP is a consultant for Merck, Moderna, Pfizer, Dynavax, Hoopika, and GSK, and has a sponsored research program with Moderna and Merck in the area of cytomegalovirus (CMV) vaccines. AG is a co-PI on COVID-vaccine studies funded through the SAMRC by the HIV vaccine trials network, EDCTP, ANRS, ImmunityBio, Janssen, and Moderna. AB and EJF receive funding from IMPAACT and AB receives funding from Unitaid for trials evaluating non-bNAb approaches to maternal-infant HIV prophylaxis. CKC participated in a RSV advisory board for Sanofi and previously received grants from Gilead, paid to her University, for clinical research. SM, KC, PEF, DS, and VMK are employed by IAVI, a non-profit organization that is pursuing the development of a combination anti-HIV bNAb product. This does not alter our adherence to PLOS ONE policies on sharing data and materials. No other authors report conflicts of interest.
Figures

Update of
-
Cost-effectiveness of broadly neutralizing antibodies for infant HIV prophylaxis in settings with high HIV burdens: a simulation modeling study.medRxiv [Preprint]. 2023 Nov 7:2023.11.06.23298184. doi: 10.1101/2023.11.06.23298184. medRxiv. 2023. Update in: PLoS One. 2025 Mar 19;20(3):e0318940. doi: 10.1371/journal.pone.0318940. PMID: 37986879 Free PMC article. Updated. Preprint.
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). The path that ends AIDS: UNAIDS Global AIDS Update 2023 [cited 2023 September 17]. Available from: https://www.unaids.org/sites/default/files/media_asset/2023-unaids-globa...
-
- Joint United Nations Programme on HIV/AIDS. 2021 UNAIDS Global AIDS Update – Confronting inequalities – Lessons for pandemic responses from 40 years of AIDS [cited 2022 October 6]. Available from: https://www.unaids.org/en/resources/documents/2021/2021-global-aids-update
-
- McFarland EJ, Cunningham CK, Muresan P, Capparelli EV, Perlowski C, Morgan P, et al.; International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1112 Team. Safety, tolerability, and pharmacokinetics of a long-acting broadly neutralizing human immunodeficiency virus type 1 (HIV-1) monoclonal antibody VRC01LS in HIV-1–exposed newborn infants. J Infect Dis. 2021. Dec;224(11):1916–24. doi: 10.1093/infdis/jiab229 - DOI - PMC - PubMed
-
- Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, et al.; VRC 606 Study Team. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018. Jan;15(1):e1002493. doi: 10.1371/journal.pmed.1002493 - DOI - PMC - PubMed

