Role of tristability in the robustness of the differentiation mechanism
- PMID: 40106426
- PMCID: PMC11922266
- DOI: 10.1371/journal.pone.0316666
Role of tristability in the robustness of the differentiation mechanism
Abstract
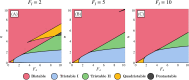
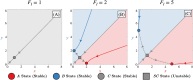
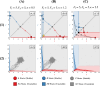
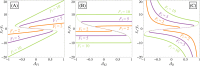
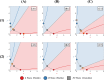
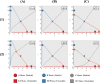
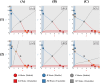
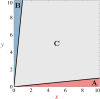
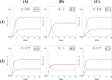
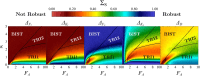
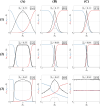
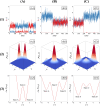
During cell differentiation, identical pluripotent cells undergo a specification process marked by changes in the expression of key genes, regulated by transcription factors that can inhibit the transcription of a competing gene or activate their own transcription. This specification is orchestrated by gene regulatory networks (GRNs), encompassing transcription factors, biochemical reactions, and signalling cascades. Mathematical models for these GRNs have been proposed in various contexts, to replicate observed robustness in differentiation properties. This includes reproducible proportions of differentiated cells with respect to parametric or stochastic noise and the avoidance of transitions between differentiated states. Understanding the GRN components controlling these features is crucial. Our study thoroughly explored an extended version of the Toggle Switch model with auto-activation loops. This model represents cells evolving from common progenitors in one out of two fates (A or B, bistable regime) or, additionally, remaining in their progenitor state (C, tristable regime). Such a differentiation into populations with three distinct cell fates is observed during blastocyst formation in mammals, where inner cell mass cells can remain in that state or differentiate into epiblast cells or primitive endoderm. Systematic analysis revealed that the existence of a stable non-differentiated state significantly impacts the GRN's robustness against parametric variations and stochastic noise. This state reduces the sensitivity of cell populations to parameters controlling key gene expression asymmetry and prevents cells from making transitions after acquiring a new identity. Stochastic noise enhances robustness by decreasing sensitivity to initial expression levels and helping the system escape from the non-differentiated state to differentiated cell fates, making the differentiation more efficient.
Copyright: © 2025 Robert et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Gata6, Nanog and Erk signaling control cell fate in the inner cell mass through a tristable regulatory network.Development. 2014 Oct;141(19):3637-48. doi: 10.1242/dev.109678. Epub 2014 Sep 10. Development. 2014. PMID: 25209243
-
Cell Fate Specification Based on Tristability in the Inner Cell Mass of Mouse Blastocysts.Biophys J. 2016 Feb 2;110(3):710-722. doi: 10.1016/j.bpj.2015.12.020. Biophys J. 2016. PMID: 26840735 Free PMC article.
-
Does mouse embryo primordial germ cell activation start before implantation as suggested by single-cell transcriptomics dynamics?Mol Hum Reprod. 2016 Mar;22(3):208-25. doi: 10.1093/molehr/gav072. Epub 2016 Jan 5. Mol Hum Reprod. 2016. PMID: 26740066
-
Making lineage decisions with biological noise: Lessons from the early mouse embryo.Wiley Interdiscip Rev Dev Biol. 2018 Jul;7(4):e319. doi: 10.1002/wdev.319. Epub 2018 Apr 30. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29709110 Free PMC article. Review.
-
Maintaining differentiated cellular identity.Nat Rev Genet. 2012 May 18;13(6):429-39. doi: 10.1038/nrg3209. Nat Rev Genet. 2012. PMID: 22596319 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

