A methodological assessment of randomization integrity in alteplase for acute ischemic stroke individual patient data meta-analyses
- PMID: 40106441
- PMCID: PMC11922233
- DOI: 10.1371/journal.pone.0315342
A methodological assessment of randomization integrity in alteplase for acute ischemic stroke individual patient data meta-analyses
Abstract
Objectives: Little is known about the integrity of randomization for randomized controlled trials (RCT) included in alteplase for stroke meta-analyses. If the RCTs were not properly randomized, the results could not be accepted at face value. The objective was to assess the integrity of randomization in individual patient data (IPD) meta-analyses supporting alteplase for acute ischemic stroke.
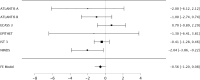
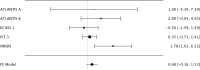
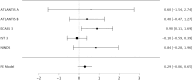
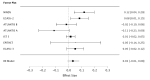
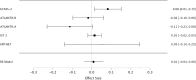
Methods: We assessed randomization reporting, performed qualitative risk of bias assessments arising from the randomization process, and performed fixed effects meta-analyses of baseline variables for which zero heterogeneity is expected if all included RCTs have unbiased randomization. Fixed-effects meta-analyses of baseline age, weight, and National Institute of Health Stroke Scale (NIHSS) score were performed. If heterogeneity was present (I2 > 0%), trials were systematically removed, starting with the RCT with the largest t-statistic until the I2 value was 0%.
Results: The NINDS rt-PA Stroke Study had a high risk of bias, the ECASS-3 RCT had some concerns, and all other trials were graded as low risk according to the Cochrane Risk of Bias (ROB-2) tool. The NINDS rt-PA Stroke Study contributed to heterogeneity in age and weight meta-analyses, and the ECASS-3 RCT contributed to heterogeneity in the NIHSS score meta-analysis. Removal of suspect trials resulted in the expected I2 value of 0%.
Conclusions: The NINDS rt-PA Stroke Study and ECASS-3 trials contributed to heterogeneity in fixed effects meta-analyses of baseline variables while there should have been none. These RCTs are likely a source of selection bias in IPD meta-analyses due to suspect randomization.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al.. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418. - PubMed
-
- Borzak S, Ridker PM. Discordance between meta-analyses and large-scale randomized, controlled trials: examples from the management of acute myocardial infarction. American College of Physicians. 1995, p. 873–7. - PubMed
-
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al.. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 2019;366. - PubMed
-
- Berger V. Selection bias and covariate imbalances in randomized clinical trials. John Wiley & Sons; 2007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

