Unveiling the Drivers of Polio Vaccine Uptake: Insights from a Multi-Country Study of 37 Nations in Sub-Saharan Africa
- PMID: 40106450
- PMCID: PMC11922275
- DOI: 10.1371/journal.pone.0316884
Unveiling the Drivers of Polio Vaccine Uptake: Insights from a Multi-Country Study of 37 Nations in Sub-Saharan Africa
Abstract
Background: Childhood vaccination is a highly cost-effective strategy for preventing vaccine-preventable diseases, including poliomyelitis. Despite advancements in vaccination coverage across Africa, polio remains a public health concern. Limited multi-country analyses on oral polio vaccine (OPV) dropout in African nations hinder the development of context-specific interventions. This study investigates OPV uptake and associated factors in sub-Saharan Africa (SSA).
Methods: This study analyzed data from the Demographic and Health Surveys of 37 sub-Saharan African countries, encompassing 60,846 children aged 12-23 months. Multilevel multinomial logistic regression models were employed to explore associations between individual- and community-level factors and vaccination status, categorized as non-vaccinated, dropout, or fully vaccinated. Four nested models were assessed, with the model exhibiting the lowest deviance (-2 Log-likelihood Ratio (-2LLR)) identified as the best fit. Variables with p-values < 0.2 in bivariable analysis were included in the multivariable analysis. The adjusted Relative Risk Ratios (aRRR) with 95% Confidence Intervals (CI) were reported to determine statistical significance and the strength of associations.
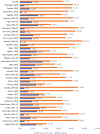
Results: Among children aged 12-23 months, OPV1, OPV2, and OPV3 coverage rates were 86.59%, 81.27%, and 68.41%, respectively. The prevalence of OPV dropout and full vaccination in SSA were 19.38% (95% CI: 19.06%, 19.69%) and 67.77% (95% CI: 67.40%, 68.14%), respectively, with a dropout rate of 20.98%. Key factors significantly associated with non-vaccination included maternal education (primary: aRRR = 0.58; secondary: aRRR = 0.64; higher: aRRR = 0.75), household wealth (poorer: aRRR = 0.91; middle: aRRR = 0.82; richer: aRRR = 0.70), maternal age (20-29: aRRR = 0.67; 30-39: aRRR = 0.60; 40-49: aRRR = 0.59), health facility delivery (aRRR = 0.28), media exposure (aRRR = 0.64), marital status (currently married: aRRR = 0.87), parity (2-3 births: aRRR = 1.11), and rural residence (aRRR = 0.73). Regional disparities revealed higher risks of non-vaccination and dropout in Southern, Central, and West Africa compared to East Africa.
Conclusion: This study highlights the multifaceted determinants of oral polio vaccination dropout in SSA. Targeted interventions, such as improving maternal education, enhancing access to healthcare facilities, addressing socioeconomic inequalities, and mitigating regional disparities, are essential to boosting vaccination coverage and preventing polio resurgence. Focused efforts in Western and Central Africa are critical to sustaining and expanding vaccination programs.
Copyright: © 2025 Antehunegn Tesema et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Complete basic childhood vaccination and associated factors among children aged 12-23 months in East Africa: a multilevel analysis of recent demographic and health surveys.BMC Public Health. 2020 Dec 1;20(1):1837. doi: 10.1186/s12889-020-09965-y. BMC Public Health. 2020. PMID: 33256701 Free PMC article.
-
What is causing high polio vaccine dropout among Pakistani children?Public Health. 2018 Nov;164:16-25. doi: 10.1016/j.puhe.2018.07.008. Epub 2018 Aug 25. Public Health. 2018. PMID: 30153528
-
Demand-side determinants of timely vaccination of oral polio vaccine in social mobilization network areas of CORE Group polio project in Uttar Pradesh, India.BMC Infect Dis. 2018 May 16;18(1):222. doi: 10.1186/s12879-018-3129-2. BMC Infect Dis. 2018. PMID: 29769034 Free PMC article.
-
Barriers to childhood immunization in sub-Saharan Africa: A systematic review.BMC Public Health. 2020 Jul 14;20(1):1108. doi: 10.1186/s12889-020-09169-4. BMC Public Health. 2020. PMID: 32664849 Free PMC article.
-
Uptake and determinants of routine immunization among vulnerable children and adolescents in sub-Saharan Africa: A scoping review.Vaccine. 2025 Apr 30;54:127021. doi: 10.1016/j.vaccine.2025.127021. Epub 2025 Mar 20. Vaccine. 2025. PMID: 40117940
References
-
- Ozawa S, Clark S, Portnoy A. Return on investment from childhood immunization in low- and middle-income countries, 2011-20. Health Affairs. 2016. - PubMed
-
- Brickley EB, Wieland-Alter W, Connor RI, Ackerman ME, Boesch AW, Arita M, et al.. Intestinal immunity to poliovirus following sequential trivalent inactivated polio vaccine/bivalent oral polio vaccine and trivalent inactivated polio vaccine-only immunization schedules: analysis of an open-label, randomized, controlled trial in chilean infants. Clin Infect Dis. 2018;67(suppl_1):S42–50. doi: 10.1093/cid/ciy603 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials