Evidence for infectious merozoites of Plasmodium falciparum from natural isolates of cultured hepatoma cells infected with sporozoites
- PMID: 40106488
- PMCID: PMC11922282
- DOI: 10.1371/journal.pone.0319901
Evidence for infectious merozoites of Plasmodium falciparum from natural isolates of cultured hepatoma cells infected with sporozoites
Abstract
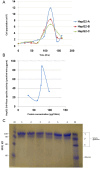
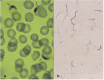
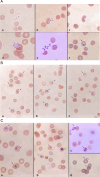
Previous cell culture systems using various human hepatoma cell lines established that the intra-hepatic stages of Plasmodium falciparum could be studied ex vivo. However, only one of these culture systems yielded infective merozoites that subsequently completed the parasite's life cycle outside a human host. We hypothesized that a major limitation is the use of laboratory-adapted P. falciparum blood stages for sporozoites generation. Plasmodium falciparum sporozoites were generated by membrane-feeding of gametocyte-infected blood samples from hospital patients to Anopheles arabiensis. Subsequently, cultured HepG2 cells were infected with the sporozoites. From 6 days post-sporozoite inoculation, liver merozoites could be harvested from the cell supernatants. When co-cultured with O + erythrocytes, these merozoites established a blood infection and yielded erythrocytic stage parasites that re-infected erythrocytes. To confirm that the erythrocytic parasites generated were P. falciparum, RNA expressed by the erythrocytic parasites was isolated and used as control in microarray analysis against RNA expressed by irradiated erythrocytic parasites; subsequently, P. falciparum genes were identified. The cultured HepG2 cells permitted the full intra-hepatic maturation of P. falciparum parasites from natural isolates. Infective merozoites were yielded which gave rise to the erythrocytic stage P. falciparum post-infection into O + erythrocytes. The full intra-hepatic maturation of the naturally isolated P. falciparum parasites in a HepG2 cell culture system is possible. This finding has important implications for malaria research and vaccine development.
Copyright: © 2025 Adeyemi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
AMA1 and MAEBL are important for Plasmodium falciparum sporozoite infection of the liver.Cell Microbiol. 2017 Sep;19(9). doi: 10.1111/cmi.12745. Epub 2017 May 18. Cell Microbiol. 2017. PMID: 28371168
-
Culture of exoerythrocytic stages of the malaria parasites Plasmodium falciparum and Plasmodium vivax.Methods Mol Biol. 2009;470:263-73. doi: 10.1007/978-1-59745-204-5_18. Methods Mol Biol. 2009. PMID: 19089388
-
Progress in imaging methods: insights gained into Plasmodium biology.Nat Rev Microbiol. 2017 Jan;15(1):37-54. doi: 10.1038/nrmicro.2016.158. Epub 2016 Nov 28. Nat Rev Microbiol. 2017. PMID: 27890922 Review.
-
Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity.Nat Med. 2003 Jan;9(1):93-6. doi: 10.1038/nm808. Epub 2002 Dec 16. Nat Med. 2003. PMID: 12483205
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
References
-
- World malaria report 2022. Geneva: World Health Organization. 2022; Licence: CC BY-NC-SA 3.0 IGO.
MeSH terms
LinkOut - more resources
Full Text Sources

