Transfer RNA acetylation regulates in vivo mammalian stress signaling
- PMID: 40106564
- PMCID: PMC11922055
- DOI: 10.1126/sciadv.ads2923
Transfer RNA acetylation regulates in vivo mammalian stress signaling
Abstract
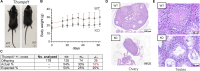
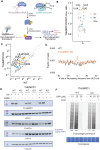
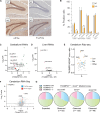
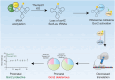
Transfer RNA (tRNA) modifications are crucial for protein synthesis, but their position-specific physiological roles remain poorly understood. Here, we investigate the impact of N4-acetylcytidine (ac4C), a highly conserved tRNA modification catalyzed by the essential acetyltransferase Nat10. By targeting Thumpd1, a nonessential adapter protein required for Nat10-catalyzed tRNA acetylation, we determine that loss of tRNA acetylation leads to reduced levels of tRNALeu, increased ribosome stalling, and activation of eIF2α phosphorylation. Thumpd1 knockout mice exhibit growth defects and sterility. Concurrent knockout of Thumpd1 and the stress-sensing kinase Gcn2 causes penetrant postnatal lethality in mice, indicating a critical genetic interaction. Our findings demonstrate that a modification restricted to a single position within type II cytosolic tRNAs can regulate ribosome-mediated stress signaling in mammalian organisms, with implications for our understanding of translational control and therapeutic interventions.
Figures


Update of
-
Transfer RNA acetylation regulates in vivo mammalian stress signaling.bioRxiv [Preprint]. 2024 Jul 28:2024.07.25.605208. doi: 10.1101/2024.07.25.605208. bioRxiv. 2024. Update in: Sci Adv. 2025 Mar 21;11(12):eads2923. doi: 10.1126/sciadv.ads2923. PMID: 39091849 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

