Prognostic role of interim PET in follicular lymphoma: a post hoc study of FOLL12 trial by Fondazione Italiana Linfomi
- PMID: 40106688
- PMCID: PMC12182788
- DOI: 10.1182/bloodadvances.2024014790
Prognostic role of interim PET in follicular lymphoma: a post hoc study of FOLL12 trial by Fondazione Italiana Linfomi
Abstract
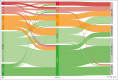
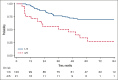
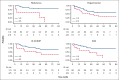
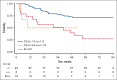
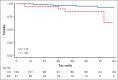
We analyzed metabolic response using interim positron emission tomography scan (iPET) in a subset of patients with follicular lymphoma (FL) enrolled in the randomized FOLL12 trial. Patients with grade 1-3a FL with an iPET performed between cycles 4 and 5 of first-line immunochemotherapy (ICT) were included; PET scan had to be centrally reviewed for the definition of Deauville score (DS) and were considered positive for DS 4-5. Overall 123 patients out of 211 with iPET were available for central review. Of these, 43% were older than 60, 33% had high-risk FLIPI2, and 47% received rituximab-bendamustine as the induction regimen. iPET showed a complete metabolic response (CMR) in 83% of cases. CMR at the end-of-induction therapy PET scan (eoiPET) was confirmed in 91% of iPET-negative patients. The 5-year progression-free survival (PFS) was 70% for iPET-negative and 34% for iPET-positive cases. In multivariate analysis, positive iPET was an independent prognostic factor for PFS. Combining iPET and eoiPET, the 3-year PFS was 78% for both negative iPET and eoiPET, with a reduced risk of progression compared to double-positive iPET/eoiPET cases. The 5-year overall survival rate was 96% for iPET-negative and 85% for DS 4-5. Our results confirm that iPET in patients with FL treated with standard ICT is a strong prognostic factor. Assessment of early metabolic response in FL may be considered for defining a novel generation of early response-adapted trials in FL. This trial was registered at www.ClinicalTrials.gov as #NCT02063685.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Freedman A, Jacobsen E. Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol. 2020;95(3):316–327. - PubMed
-
- Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the International Follicular Lymphoma Prognostic Factor Project. J Clin Oncol. 2009;27(27):4555–4562. - PubMed

