Comprehensive genomic profiling by liquid biopsy portrays metastatic colorectal cancer mutational landscape to predict antitumor efficacy of FOLFIRI plus cetuximab in the CAPRI-2 GOIM trial
- PMID: 40107157
- PMCID: PMC11964631
- DOI: 10.1016/j.esmoop.2025.104511
Comprehensive genomic profiling by liquid biopsy portrays metastatic colorectal cancer mutational landscape to predict antitumor efficacy of FOLFIRI plus cetuximab in the CAPRI-2 GOIM trial
Abstract
Background: Limited evidence is currently available on the role of liquid biopsy (LBx) in predicting the efficacy of anti-epidermal growth factor receptor (EGFR) therapies in metastatic colorectal cancer (mCRC).
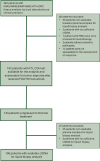
Methods: The CAPRI-2 GOIM is a phase II trial investigating the use of LBx-comprehensive genomic profiling (CGP)-guided, cetuximab-based treatment through three subsequent lines of therapy in patients with RAS/BRAF wild-type (WT) mCRC. LBx-CGP is carried out at baseline and at progressive disease to first- and second-line therapies. In case of RAS/BRAF WT circulating tumor DNA at progressive disease, EGFR therapeutic blockade is continued by combining cetuximab with a different chemotherapy backbone. The primary endpoint is overall response rate (ORR) by RECIST 1.1 criteria. Tumor molecular characteristics by LBx-CGP are correlated with treatment efficacy.
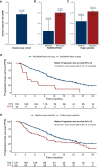
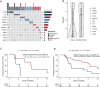
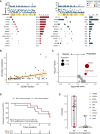
Results: One hundred and ninety-two RAS/BRAF WT microsatellite stable mCRC patients treated with FOLFIRI plus cetuximab with baseline LBx-CGP and assessable for response were included in the analysis. One hundred and thirty-seven patients with WT tumors for potential anti-EGFR drug resistance genes (RAS/BRAF/EGFR/PIK3CA/MAP2K1/MET/RET/ALK/ROS1/NTRK/NF1/FGFR, and HER2 amplification; 'negatively hyper-selected' cases) had 78.1% ORR compared with 54.5% ORR for patients with mutations [odds ratio 2.95, 95% confidence interval (CI) 1.44-6.10, P = 0.001]. 'Negatively hyper-selected' patients had median progression-free survival of 12.35 months (95% CI 10.58-15.4 months) compared with 8.68 months (95% CI 4.87-12.1 months) for patients with mutations (hazard ratio 0.64, 95% CI 0.44-0.92, P = 0.017). High cancer cell clonality of pathogenic variants (PVs) correlated with worse median progression-free survival (3.55 months, 95% CI 2.57 months to NE) compared with low cancer cell clonality of PV (9.63 months, 95% CI 7.16 months to NE, P = 0.21). After first-line therapy failure, approximately one out of five patients had acquired PVs of potential anti-EGFR drug resistance genes, whereas RAS/BRAF WT circulating tumor DNA was maintained in most patients (78.5%).
Conclusions: These results support the integration of LBx-CGP for implementing the efficacy and for optimizing the use of anti-EGFR therapies in RAS/BRAF WT mCRC.
Keywords: cetuximab; colorectal cancer; comprehensive genomic profiling; liquid biopsy.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Ciardiello F., Ciardiello D., Martini G., Napolitano S., Tabernero J., Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin. 2022;72(4):372–401. - PubMed
-
- Cervantes A., Adam R., Roselló S., et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32. - PubMed
-
- Ciardiello F., Normanno N., Maiello E., et al. Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next-generation sequencing: findings from the CAPRI-GOIM trial. Ann Oncol. 2014;25(9):1756–1761. - PubMed
-
- Randon G., Maddalena G., Germani M.M., et al. Negative ultraselection of patients with RAS/BRAF wild-type, microsatellite-stable metastatic colorectal cancer receiving anti-EGFR-based therapy. JCO Precis Oncol. 2022;6 doi: 10.1200/PO.22.00356. [Erratum in: JCO Precis Oncol. 2022 Jul;6:e2200356. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

