Safety and efficacy of combined treatment with tumor-infiltrating lymphocytes and oncolytic adenovirus TILT-123 in metastatic melanoma
- PMID: 40107242
- PMCID: PMC11970381
- DOI: 10.1016/j.xcrm.2025.102016
Safety and efficacy of combined treatment with tumor-infiltrating lymphocytes and oncolytic adenovirus TILT-123 in metastatic melanoma
Abstract
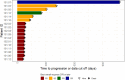
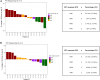
Tumor-infiltrating lymphocytes (TILs) are effective in the treatment of metastatic melanoma (MM), but toxicity limits its application. TILT-123 (igrelimogene litadenorepvec) is an oncolytic adenovirus producing interleukin-2 (IL-2) and tumor necrosis factor (TNF) upon replication. In this phase 1 trial, 17 patients with metastatic checkpoint inhibitor-resistant melanoma are treated with TILT-123 and TILs without preconditioning chemotherapy or postconditioning IL-2. The treatment is safe and feasible. According to computed tomography (CT), the objective response rate is 11.7% (2/17) and disease control is observed in 35% (6/17), including a partial response lasting >8 months and a durable complete response in a mucosal melanoma patient. According to positron emission tomography (PET), disease control is observed in 7/15 (47%) with minor or partial responses in 4/15 (27%). In the initial TILT-123 monotherapy phase of the trial, disease control is observed in 6/17 (35%) and 10/16 (63%) in CT and PET, respectively. The study demonstrates good tolerability and preliminary efficacy.
Keywords: TIL therapy; cancer immunotherapy; combination immunotherapy; cutaneous melanoma; mucosal melanoma; oncolytic virus therapy; tumor-infiltrating lymphocytes; uveal melanoma.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests T.J.M. has been a co-investigator in a trial from TILT Biotherapeutics. E.E. has received personal payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Novartis, Merck, Bristol Myers Squibb, and Pierre Fabre. She has received support for attending meetings and/or travel from Pierre Fabre and Merck. M.D. is an advisor of Achilles Therapeutics and expert group member of Guidepoint LLC and AlphaSights. R.L.E. received institutional drug funding from BMS to investigator-initiated trial. T.H.B. has received personal honoraria for lectures from Bristol Myers Squibb. C.K. is an employee and shareholder at TILT Biotherapeutics. J.M.S. is an employee and stocks/share holder at TILT Biotherapeutics. He received a research grant from TILT Biotherapeutics. He is a co-inventor of patent applications for TILT Biotherapeutics. He received personal payment for consultancy for TILT Biotherapeutics. J.H.A.C. is an employee and shareholder at TILT Biotherapeutics. L.H. is an employee and option holder at TILT Biotherapeutics. D.C.A.Q. is an employee and shareholder at TILT Biotherapeutics. R.H. is an employee at TILT Biotherapeutics. S.S. is an employee and option holder at TILT Biotherapeutics. V.C.-C. is an employee and shareholder at TILT Biotherapeutics. A.H. is a shareholder in Circio Holding ASA and an employee and shareholder at TILT Biotherapeutics Oy. I.M.S. has received personal payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, advisory board, or educational events from Bristol Myers Squibb, Instil Bio, Mendus, MSD, Novartis, Sanofi, and Takeda. She has stocks/shares in IO Biotech. She has received research grants from Adaptimmune, Asgard Therapeutics, Enara Bio, IO Biotech, Lytix Biopharma, and TILT Biotherapeutics. She has been principal investigator in trials from BMS, Immunocore, Lytix Biopharma, MSD, Novartis, Roche, and TILT Biotherapeutics. The TILT technology is patented by the company TILT Biotherapeutics.
Figures




References
-
- Kwong M.L.M., Yang J.C. Lifileucel: FDA-approved T-cell therapy for melanoma. The Oncologist. 2024;29:648–650. https://www.fda.gov/news-events/press-announcement - PMC - PubMed
-
- Chesney J.A., Ribas A., Long G.V., Kirkwood J.M., Dummer R., Puzanov I., Hoeller C., Gajewski T.F., Gutzmer R., Rutkowski P., et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined with Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2023;41:528–540. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

