Risk factors for drug-resistant pathogens in community-acquired pneumonia: systematic review and meta-analysis
- PMID: 40107661
- PMCID: PMC11920891
- DOI: 10.1183/16000617.0183-2024
Risk factors for drug-resistant pathogens in community-acquired pneumonia: systematic review and meta-analysis
Abstract
Introduction: Community-acquired pneumonia (CAP) is a leading cause of death worldwide. Reducing inappropriate and excessive use of extended-spectrum antibiotics is essential for treating CAP effectively. Evaluating the risk of drug-resistant pathogens (DRPs) is crucial for determining initial antibiotic therapy in clinical settings.
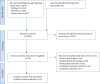
Methods: This systematic review and meta-analysis assessed the risk factors for DRPs in patients with CAP. CAP-DRPs were defined as pathogens resistant to commonly used antibiotics for CAP, including nonpseudomonal β-lactams such as ceftriaxone or sulbactam-ampicillin, macrolides and respiratory fluoroquinolones. The studies included were divided into two cohorts, namely an all-patient cohort, comprising both culture-positive and culture-negative patients, and a culture-positive pneumonia cohort, comprising patients with identified causative pathogens. The primary objective of this study was to evaluate the risk factors for CAP-DRPs in the all-patient cohort.
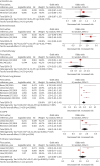
Results: 24 articles were included with 11 categorised into the all-patient cohort. The meta-analysis identified 11 significant risk factors for CAP-DRPs, namely prior DRP infection/colonisation, tracheostomy, severe respiratory failure requiring early induction of mechanical ventilation, prior use of antibiotics, chronic lung disease, COPD, wound care, neurological disorders, prior hospitalisation, nursing home residence and low activities of daily living.
Conclusion: To our knowledge, this is the first systematic review focused on CAP-DRP. Unlike previous reviews, the all-patient and culture-positive pneumonia cohorts were analysed separately. Findings from the all-patient cohort are particularly relevant for guiding initial antimicrobial selection in clinical practice. Furthermore, the abovementioned factors should be considered when developing prediction models for CAP-DRPs.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: N. Nakagawa, M. Katsurada, Y. Fukuda, S. Noguchi, N. Horita and M. Miki have nothing to disclose. H. Tsukada reports payment or honoraria for lectures, presentations, manuscript writing or educational events from MSD Ltd., Kyorin Pharma Ltd., and Meiji Sika Ltd. K. Senda has nothing to disclose. Y. Shindo reports grants from Japan Society for the Promotion of Science; payment or honoraria for lectures, presentations, manuscript writing or educational events from KYORIN Pharmaceutical Co., Ltd., AstraZeneca K.K., Nippon Boehringer Ingelheim Co., Ltd., Gilead Sciences Inc., MSD K.K., GlaxoSmithKline plc., Insmed, Inc. and Asahi Kasei Pharma Co., Ltd.; and participation on a data safety monitoring board or advisory board with GlaxoSmithKline Biologicals SA. H. Mukae reports grants from Taiho Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Taisho Pharma Co., Ltd., and Meiji Seika Pharma Co., Ltd.; payment or honoraria for lectures, presentations, manuscript writing or educational events from Pfizer Inc., MSD Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Insmed Incorporated, Gilead Sciences Inc., SHIONOGI Co., Ltd., AstraZeneca K.K., Kyorin Pharmaceutical Co., Ltd., Asahi Kasei Pharma Corporation and Chugai Pharmaceutical Co., Ltd.; and payment for expert testimony from Pfizer Inc., MSD Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Asahi Kasei Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Insmed Incorporated, Gilead Sciences Inc., and SHIONOGI Co., Ltd.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
