Liberation from mechanical ventilation using Extubation Advisor Decision Support (LEADS): protocol for a multicentre pilot trial
- PMID: 40107679
- PMCID: PMC11927467
- DOI: 10.1136/bmjopen-2024-093853
Liberation from mechanical ventilation using Extubation Advisor Decision Support (LEADS): protocol for a multicentre pilot trial
Abstract
Introduction: Timely successful liberation from invasive ventilation has the potential to minimise critically ill patients' exposure to invasive ventilation, save costs and improve outcomes; yet no trials have evaluated strategies to better inform extubation decision-making. The Liberation from mechanical ventilation using Extubation Advisor (EA) Decision Support (LEADS) Pilot Trial will assess the feasibility of a trial of a novel extubation decision support tool on feasibility metrics. The primary feasibility outcome will reflect our ability to recruit the desired population. Secondary feasibility outcomes will assess rates of (1) consent, (2) randomisation, (3) intervention adherence, (4) bidirectional crossovers and the (5) completeness of clinical outcomes collected. We will also evaluate physicians' perceptions of the usefulness of the EA tool and measure costs related to EA implementation.
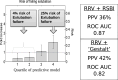
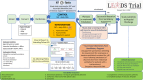
Methods and analysis: We will include critically ill adults who are invasively ventilated for ≥48 hours and who are ready to undergo a spontaneous breathing trial (SBT) with a view to extubation. Patients in the intervention arm will undergo an EA assessment that measures respiratory rate variability to derive an estimate of extubation readiness. Treating clinicians (respiratory therapists, attending physicians and intensive care unit fellows) will receive an EA report for each SBT conducted. The EA report will assist, rather than direct, extubation decision-making. Patients in the control arm will receive standard care. SBTs will be directed by clinicians, using current best evidence, without EA assessments or reports. We aim to recruit 1 to 2 patients/month in approximately 10 centres, and to achieve >75% consent rate, >95% randomisation among consented patients, >80% of EA reports generated and delivered (intervention arm), <10% crossovers (both arms) and >90% of patients with complete clinical outcomes. We will also report physician point-of-care perceptions of the usefulness of the EA tool.
Ethics and dissemination: The LEADS Pilot Trial is approved by the Research Ethics Boards of all participating centres and Clinical Trials Ontario (4008). We will disseminate the LEADS trial findings through conference presentations and publication.
Trial registration number: NCT05506904.
Protocol version: 24 April 2024.
Keywords: Adult intensive & critical care; Artificial Intelligence; Machine Learning.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: ‘Yes, there are competing interests for one or more authors and I have provided a Competing Interests statement in my manuscript and in the box below’
Figures
References
-
- Burns KEA, Jacob SK, Aguirre V, et al. Stakeholder Engagement in Trial Design: Survey of Visitors to Critically Ill Patients Regarding Preferences for Outcomes and Treatment Options during Weaning from Mechanical Ventilation. Ann Am Thorac Soc. 2016;13:1962–8. doi: 10.1513/AnnalsATS.201606-445OC. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
