Association of dietary fatty acids with longitudinal change in plasma-based biomarkers of Alzheimer's disease
- PMID: 40107919
- PMCID: PMC12094269
- DOI: 10.1016/j.tjpad.2025.100117
Association of dietary fatty acids with longitudinal change in plasma-based biomarkers of Alzheimer's disease
Abstract
Background: Elevated intake of omega-3 polyunsaturated fatty acids is linked to a reduced risk of dementia in some prospective studies. However, few studies have examined the relationship between nutrient intake and plasma biomarkers of Alzheimer's disease.
Objectives: We explored whether omega-3, omega-6, and monounsaturated fat intakes were associated with changes in plasma biomarkers of Alzheimer's disease over time.
Design: The Washington Heights-Inwood Columbia Aging Project is a prospective cohort study (1994-2021); the data set used here includes a mean follow-up of 7.0 years.
Setting: Community-based in New York City.
Participants: 599 dementia-free individuals at baseline who completed a 61-item food frequency questionnaire and had biomarkers measured in plasma from at least two different time points.
Measurements: Fatty acid intake tertiles were computed from participant-completed 61-item Willett semi-quantitative food frequency questionnaires (Channing Laboratory, Cambridge, Massachusetts) obtained once at their baseline visit. Plasma-based biomarker assays were performed, using the single molecule array technology Quanterix Simoa HD-X platform, at baseline and follow-up visits. Generalized Estimating Equations (GEE) models were used to evaluate the association between baseline nutrient intake tertile and changes in biomarkers including phospho-tau181, amyloid-beta 42/40 ratio, phospho-tau181/amyloid-beta42 ratio, glial fibrillary acidic protein, neurofilament light chain, and two biomarker patterns derived from Principal Component Analysis (PCA1 and PCA2), with higher scores indicating a high level of neurodegeneration and low level of Alzheimer's disease burden, respectively). Models were adjusted for age, sex, race/ethnicity, education, and calculated total energy intake initially, and additionally for cerebrovascular risk factors.
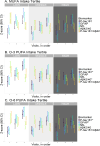
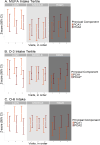
Results: Higher baseline omega-3 intake tertile was associated with lesser decline in PCA2 (β = 0.221, p < 0.001) and amyloid-beta 42/40 ratio (β = 0.022, p = 0.003), and a lesser rise in phospho-tau181 (β = -0.037, p = 0.001). Higher omega-6 intake tertile was linked to a lesser rise in phospho-tau181 (β = -0.050, p < 0.001) and glial fibrillary acidic protein (β = -0.028, p = 0.002). Most associations persisted after adjusting for cardiovascular risk factors.
Conclusions: Higher relative baseline intake of omega-3 and omega-6 fatty acids is associated with lesser progression of blood-based biomarkers of Alzheimer's disease. Consuming healthy fatty acids may help prevent accumulation of Alzheimer's disease-related pathological changes.
Keywords: Alzheimer's disease; Amyloid; Blood-based biomarker; Diet; Omega-3.
Copyright © 2025 The Author(s). Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of compting interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Brickman reports grants from NIH, during the conduct of the study; personal fees from Cedara, personal fees from Cogstate, personal fees from Cognito Therapeutics, personal fees from Cognition Therapeutics, personal fees from IQVIA, personal fees from International Neuropsychological Society, other from University of Illinois, Urbana-Champaign, other from Section editor, Alzheimer's & Dementia, outside the submitted work; In addition, Dr. Brickman has a patent US Patent # 9,867,566 issued, and a patent US Patent# 20,230,298,170 pending. Dr. Dage reports personal fees and other from Genotix Biotechnologies Inc, personal fees and other from AlzPath Inc, personal fees and other from Monument Biosciences, personal fees from Gates Ventures, personal fees from Karuna Therapeutics, personal fees from Cognito, personal fees and other from AbbVie, personal fees and other from Eisai, personal fees and other from Prevail Therapeutics, personal fees from Dolby Ventures, personal fees from Alzheimer's disease drug discovery fund, personal fees, non-financial support and other from Eli Lilly and Company, personal fees from Lab Corp, outside the submitted work; In addition, Dr. Dage has a patent COMPOUNDS AND METHODS TARGETING HUMAN TAU pending, and a patent Prevention of axonal damage using antibody binding to amyloid beta 1–42 pending. Dr. Manly reports grants from NINNIH P30 AG059303 ROl AG054070 NINDS/NIH UOl NS041588; NINNIH T35 AG044303 UOl AG009740, outside the submitted work;. Dr. Honig reports grants and personal fees from Eisai, personal fees from Biogen, grants from Cognition, grants from EIP Pharma, grants from Acumen, grants from BMS, personal fees from New Amsterdam, personal fees from Prevail/ Lilly, grants from Ferrer, grants from Vaccinex, personal fees from Genentech/Roche, personal fees from Vigil, grants from Transposon, grants from GSK, grants from UCB, personal fees from Medscape, outside the submitted work;. All other authors have nothing to disclose.
Figures
References
-
- Román G.C., Jackson R.E., Gadhia R., Román A.N., Reis J. Mediterranean diet: the role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol (Paris) 2019;175(10):724–741. doi: 10.1016/j.neurol.2019.08.005. - DOI - PubMed
-
- Wei B.Z., Li L., Dong C.W., Tan C.C., Xu W. The relationship of omega-3 fatty acids with dementia and cognitive decline: evidence from prospective cohort studies of supplementation, dietary intake, and blood markers. Am. J. Clin. Nutr. 2023;117(6):1096–1109. doi: 10.1016/j.ajcnut.2023.04.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- RF1 NS041588/NS/NINDS NIH HHS/United States
- K01 AG084849/AG/NIA NIH HHS/United States
- U01 AG009740/AG/NIA NIH HHS/United States
- RF1 AG066107/AG/NIA NIH HHS/United States
- R01 AG066107/AG/NIA NIH HHS/United States
- R01 NS041588/NS/NINDS NIH HHS/United States
- P30 AG059303/AG/NIA NIH HHS/United States
- P30 AG066462/AG/NIA NIH HHS/United States
- U01 NS041588/NS/NINDS NIH HHS/United States
- R01 AG054070/AG/NIA NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- R01 AG072474/AG/NIA NIH HHS/United States
- T35 AG044303/AG/NIA NIH HHS/United States
- R01 AG059013/AG/NIA NIH HHS/United States
- R01 AG061008/AG/NIA NIH HHS/United States
- RF1 AG054070/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical