30-Day Outcomes of Real-World Elective Carotid Stenosis Treatment Using a Dual-Layer Micromesh Stent (ROADSAVER Study)
- PMID: 40107985
- PMCID: PMC11958397
- DOI: 10.1007/s00270-025-04003-z
30-Day Outcomes of Real-World Elective Carotid Stenosis Treatment Using a Dual-Layer Micromesh Stent (ROADSAVER Study)
Abstract
Purpose: Carotid artery stenting with single-layer stents carries a risk of periprocedural cerebral embolization compared to carotid endarterectomy. Dual-layer micromesh stents were designed for improved plaque coverage and sustained embolic protection. This analysis aimed to confirm the Roadsaver dual-layer micromesh stent safety in a real-world carotid artery stenting cohort.
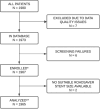
Materials and methods: ROADSAVER was a prospective, single-arm, multicenter, observational study. Patients with carotid artery stenosis, eligible for elective stenting, were enrolled at 52 sites across 13 European countries. All procedures followed standard practice. The primary outcome was the 30-day major adverse event rate, defined as the cumulative incidence of any death or stroke. All deaths, strokes, and carotid artery revascularizations were independently adjudicated.
Results: In total, 1965 patients were analysed (mean age 70.6 ± 8.8 years). Cerebral ischaemia symptoms were present in 49.4% of participants. Radial/ulnar access was used in 26.3% of cases and embolic protection in 63.8%. The 30-day major adverse event incidence was 2.2% (1.6% in asymptomatic and 2.8% in symptomatic patients), with any stroke at 1.9%, any death at 0.8%, and stroke-related death at 0.5%. Predictors of higher 30-day major adverse event risk, identified through multivariable modelling, included residual stenosis ≥ 30%, thromboembolic venous disease, previous myocardial infarction, age ≥ 75 years, family history of atherosclerosis, non-insulin-dependent diabetes mellitus, symptomatic carotid stenosis, and stent length.
Conclusion: Dual-layer micromesh carotid artery stenting is safe, with a low 30-day major adverse event incidence in real-world asymptomatic and symptomatic patients, supporting the sustained embolic protection design concept.
Level of evidence: Level 2, observational study (with dramatic effect).
Keywords: Carotid artery disease; Carotid artery revascularization; Carotid artery stenting; Cerebrovascular embolic protection; Stroke prevention.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: AS: Consultancy for Terumo; BF: Consultancy for Terumo (CAS Workshops); BN: Consultancy for Boston Scientific, Biotronik, Cook, Bentley, Penumbra; JB: Speaker for Stryker Neurovascular; Medtronic, CEC member of the Inspire-S and Inspire-A registries; J-LB: Consultancy for Terumo (CAS Workshops); KDe: Consultancy for Abbott, BD, Bentley, Biotronik, Boston Scientific, Cook, Cordis, Getinge, Gore, iVascular, Penumbra, Philips, Terumo; MP: Honoraria, institutional grants for research, clinical trials from: Abbott Vascular, Boston Scientific Corp., Gore & Associates, Inari Medical, Philips-Spectranetics, PQ-Bypass, Reflow Medical, Reva Medical, Terumo, TriReme, Veryan; OF: Consultancy for iVascular, Cerenovus, GE Healthcare; PO-P: Fees for conducting proctoring CAS procedures from Boston, Medtronic, Terumo; RB: Consultancy for Terumo; RL: Advisory board member, lecture honoraria, scientific grants from Abbott Vascular, Alvimedica, Boston Scientific, BD Bard, B.Braun, Biotronik, Contego Medical, Cardionovum, iVascular, Medtronic, Terumo; RRC: Consultancy for Boston Scientifics, Abbott Vascular; SK: Consultancy for Terumo; SM-H: Consultancy for Eurocor, Alvimedica, Terumo; TF: Consultancy for Terumo, Biotronik, BD, BSCI, Abbott. Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional Review Board of each participating centre approved the study as per local regulations. Informed Consent: Informed consent was obtained from all individual participants included in the study. Consent for Publication: Consent for publication was obtained for every individual person’s data included in the study.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

