Mitigation and Management of Common Toxicities Associated with the Administration of CAR-T Therapies in Oncology Patients
- PMID: 40108072
- PMCID: PMC12174263
- DOI: 10.1007/s40264-025-01538-5
Mitigation and Management of Common Toxicities Associated with the Administration of CAR-T Therapies in Oncology Patients
Abstract
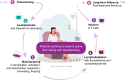
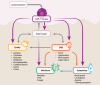
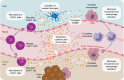
Chimeric antigen receptor T-cell (CAR-T) therapies are one of the main approaches among targeted cellular therapies. Despite the potential benefit and durable responses observed in some patients receiving CAR-T therapies, serious and potentially fatal toxicities remain a major challenge. The most common CAR-T-associated toxicities include cytokine release syndrome (CRS), neurotoxicity, cytopenias, and infections. While CRS and neurotoxicity are generally managed with tocilizumab and corticosteroids, respectively, high-grade toxicities can be life-threatening. Close postinfusion monitoring and assessment of clinical laboratory parameters, patient-related and clinical risk factors (e.g., age, tumor burden, comorbidities, baseline laboratory parameters, and underlying abnormalities), and therapy-related risk factors (e.g., CAR-T type, dose, and CAR-T-induced toxicity) are effective strategies to mitigate the toxicities. Clinical laboratory parameters, including various cytokines, have been identified for CRS (interleukin [IL]-1, IL-2, IL-5, IL-6, IL-8, IL-10, C-reactive protein [CRP], interferon [IFN]-γ, ferritin, granulocyte-macrophage colony-stimulating factor [GM-CSF], and monocyte chemoattractant protein-1), neurotoxicity (IL-1, IL-2, IL-6, IL-15, tumor necrosis factor [TNF]-α, GM-CSF, and IFN-γ), cytopenias (IL-2, IL-4, IL-6, IL-10, IFN-γ, ferritin, and CRP), and infections (IL-8, IL-1β, CRP, IFN-γ, and procalcitonin). CAR-T-associated toxicities can be monitored and treated to mitigate the risk to patients. Assessment of alterations in clinical laboratory parameter values that are correlated with CAR-T-associated toxicities may predict development and/or severity of a given toxicity, which can improve patient management strategies and ultimately enable the patients to better tolerate these therapies.
Plain language summary
Chimeric antigen receptor T-cell (CAR-T) therapies are used in the treatment of various aggressive blood cancers. These therapies use a patient’s immune cells (T cells) that are genetically modified to fight cancer. In this article, we focus on the adverse effects associated with CAR-T therapies and discuss how they can be managed. The most common CAR-T-associated adverse effects include cytokine release syndrome (a rapid release of signaling proteins [cytokines] from affected immune cells), neurotoxicity (toxic effects on the nervous system), cytopenias (lower-than-normal blood cell levels), and infections. Patients receiving CAR-T therapies need to be closely monitored for signs of any adverse effects. Some of these effects can be prevented or treated with medications. However, current efforts focus on making the adverse effects less severe, and on identifying risk factors that may predict the likelihood and onset of a potential adverse effect. When an adverse effect occurs, the levels of certain molecules in the blood change. These changes can help physicians determine the type of adverse effect and select the best treatment to combat it. Some patient features (e.g., age, medical conditions, the size and spread of the tumor, and levels of certain molecules in the blood) and treatment-related factors (e.g., therapy type and dose) should be considered before starting a CAR-T therapy. Adverse effects are monitored and treated to reduce the risk to patients. Evaluating the levels of certain parameters in the blood can improve patient management strategies and help patients better tolerate CAR-T therapies.
© 2025. GSK.
Conflict of interest statement
Declarations. Funding: This work and the related publication were sponsored by GSK. Conflicts of Interest: All authors are employees of GSK. J.R. and H.S. hold financial equities in GSK. Ethics Approval: Not applicable; no data were analyzed in this opinion paper. Consent to Participate: Not applicable. Consent for Publication: Not applicable. Availability of Data and Material: Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study. Code Availability: Not applicable. Authors’ Contribution: All authors contributed to the concept of this manuscript, and all authors reviewed the manuscript and provided final approval for publication.
Figures


Similar articles
-
Riding the storm: managing cytokine-related toxicities in CAR-T cell therapy.Semin Immunopathol. 2024 Jul 16;46(3-4):5. doi: 10.1007/s00281-024-01013-w. Semin Immunopathol. 2024. PMID: 39012374 Free PMC article. Review.
-
Cytokine Release Syndrome and Neurotoxicity Following CD19 CAR-T in B-Cell Lymphoma.Transplant Cell Ther. 2025 Jul;31(7):419-433. doi: 10.1016/j.jtct.2025.03.011. Epub 2025 Apr 25. Transplant Cell Ther. 2025. PMID: 40288610
-
CAR T-cell toxicities: from bedside to bench, how novel toxicities inform laboratory investigations.Blood Adv. 2024 Aug 27;8(16):4348-4358. doi: 10.1182/bloodadvances.2024013044. Blood Adv. 2024. PMID: 38861351 Free PMC article. Review.
-
Low Peripheral Blood Counts and Elevated Proinflammatory Cytokines Signal a Poor CD19 Chimeric Antigen Receptor T-cell Response in Acute Lymphoblastic Leukemia.Transplant Cell Ther. 2025 Aug;31(8):551-564. doi: 10.1016/j.jtct.2025.05.003. Epub 2025 May 20. Transplant Cell Ther. 2025. PMID: 40398620
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
-
- Parsonidis P, Papasotiriou I. Adoptive cellular transfer immunotherapies for cancer. Cancer Treat Res Commun. 2022;32: 100575. 10.1016/j.ctarc.2022.100575. - PubMed
-
- Kim GB, Riley JL, Levine BL. Engineering T cells to survive and thrive in the hostile tumor microenvironment. Curr Opin Biomed Eng. 2022;21:100360. 10.1016/j.cobme.2021.100360.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

