Liquid biopsies in cancer
- PMID: 40108089
- PMCID: PMC11923355
- DOI: 10.1186/s43556-025-00257-8
Liquid biopsies in cancer
Abstract
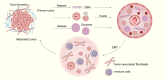
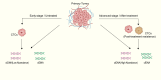
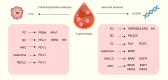
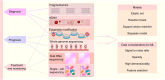
Cancer ranks among the most lethal diseases worldwide. Tissue biopsy is currently the primary method for the diagnosis and biological analysis of various solid tumors. However, this method has some disadvantages related to insufficient tissue specimen collection and intratumoral heterogeneity. Liquid biopsy is a noninvasive approach for identifying cancer-related biomarkers in peripheral blood, which allows for repetitive sampling across multiple time points. In the field of liquid biopsy, representative biomarkers include circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and exosomes. Many studies have evaluated the prognostic and predictive roles of CTCs and ctDNA in various solid tumors. Although these studies have limitations, the results of most studies appear to consistently demonstrate the correlations of high CTC counts and ctDNA mutations with lower survival rates in cancer patients. Similarly, a reduction in CTC counts throughout therapy may be a potential prognostic indicator related to treatment response in advanced cancer patients. Moreover, the biochemical characteristics of CTCs and ctDNA can provide information about tumor biology as well as resistance mechanisms against targeted therapy. This review discusses the current clinical applications of liquid biopsy in cancer patients, emphasizing its possible utility in outcome prediction and treatment decision-making.
Keywords: Circulating tumor DNA; Circulating tumor cell; Liquid biopsy; Minimal residual disease; Personalized medicine; Prognosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
