Individualized non-invasive deep brain stimulation of the basal ganglia using transcranial ultrasound stimulation
- PMID: 40108143
- PMCID: PMC11923056
- DOI: 10.1038/s41467-025-57883-7
Individualized non-invasive deep brain stimulation of the basal ganglia using transcranial ultrasound stimulation
Abstract
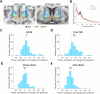
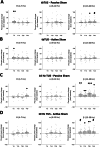
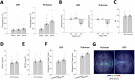
Transcranial ultrasound stimulation (TUS) offers precise, non-invasive neuromodulation, though its impact on human deep brain structures remains underexplored. Here we examined TUS-induced changes in the basal ganglia of 10 individuals with movement disorders (Parkinson's disease and dystonia) and 15 healthy participants. Local field potentials were recorded using deep brain stimulation (DBS) leads in the globus pallidus internus (GPi). Compared to sham, theta burst TUS (tbTUS) increased theta power during stimulation, while 10 Hz TUS enhanced beta power, with effects lasting up to 40 min. In healthy participants, a stop-signal task assessed tbTUS effects on the GPi, with pulvinar stimulation serving as an active sham. GPi TUS prolonged stop-signal reaction times, indicating impaired response inhibition, whereas pulvinar TUS had no effect. These findings provide direct electrophysiological evidence of TUS target engagement and specificity in deep brain structures, suggesting its potential as a noninvasive DBS strategy for neurological and psychiatric disorders.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

