Orally delivered toxin-binding protein protects against diarrhoea in a murine cholera model
- PMID: 40108169
- PMCID: PMC11923127
- DOI: 10.1038/s41467-025-57945-w
Orally delivered toxin-binding protein protects against diarrhoea in a murine cholera model
Abstract
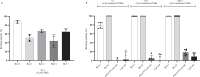
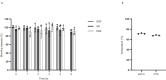
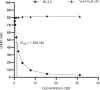
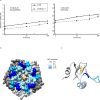
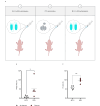
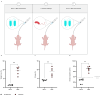
The ongoing seventh cholera pandemic, which began in 1961, poses an escalating threat to public health. There is a need for new cholera control measures, particularly ones that can be produced at low cost, for the one billion people living in cholera-endemic regions. Orally delivered VHHs, functioning as target-binding proteins, have been proposed as a potential approach to control gastrointestinal pathogens. Here, we describe the development of an orally deliverable bivalent VHH construct that binds to the B-pentamer of cholera toxin, showing that it inhibits toxin activity in a murine challenge model. Infant mice given the bivalent VHH prior to V. cholerae infection exhibit a significant reduction in cholera toxin-associated intestinal fluid secretion and diarrhoea. In addition, the bivalent VHH reduces V. cholerae colonization levels in the small intestine by a factor of 10. This cholera toxin-binding protein holds promise for protecting against severe diarrhoea associated with cholera.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: All authors affiliated with Bactolife A/S are present or past employees of Bactolife A/S. A.H.L. and S.W.T. are shareholders of Bactolife A/S. The remaining authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

