Coupling furfural oxidation for bias-free hydrogen production using crystalline silicon photoelectrodes
- PMID: 40108174
- PMCID: PMC11923221
- DOI: 10.1038/s41467-025-58000-4
Coupling furfural oxidation for bias-free hydrogen production using crystalline silicon photoelectrodes
Abstract
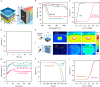
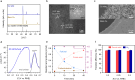
To commercialize the technology of photoelectrochemical hydrogen production, it is essential to surpass the US. Department of Energy target of 0.36 mmol h-1 cm-2 for 1-sun hydrogen production rate. In this study, we utilize crystalline silicon, which can exhibit the highest photocurrent density (43.37 mA cm-2), as the photoelectrode material. However, achieving bias-free water splitting (>1.6 V) remains challenging due to the intrinsic low photovoltage of crystalline silicon (0.6 V). To address this limitation, we replace water oxidation with low-potential furfural oxidation, enabling not only bias-free hydrogen production but also dual hydrogen production at both the cathodic and anodic sides. This approach results in a record 1-sun hydrogen production rate of 1.40 mmol h-1 cm-2, exceeding the Department of Energy target by more than fourfold.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

