Insights into GLP-1 and insulin secretion mechanisms in pasireotide-induced hyperglycemia highlight effectiveness of Gs-targeting diabetes treatment
- PMID: 40108209
- PMCID: PMC11923236
- DOI: 10.1038/s41598-025-90896-2
Insights into GLP-1 and insulin secretion mechanisms in pasireotide-induced hyperglycemia highlight effectiveness of Gs-targeting diabetes treatment
Abstract
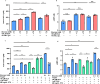
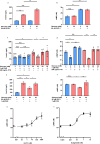
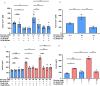
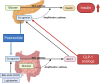
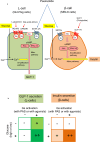
Pasireotide frequently causes severe hyperglycemia; however, its detailed mechanism remains unknown. There are no published guidelines regarding the optimal management of pasireotide-induced hyperglycemia based on its pathophysiology. Herein, we successfully switched a patient with acromegaly from a dipeptidyl peptidase-4 (DPP-4) inhibitor to a glucagon-like peptide-1 (GLP-1) analog due to pasireotide-induced deterioration of glycemic control, and we examined the underlying mechanism for glycemic control. An in vitro study was conducted using pancreatic β-cell line, MIN-6, stably expressing GLP-1R (GLP-1R-MIN-6 cells) and intestinal L-cell line, GLUTag. High glucose levels and Gs-coupled receptor stimulation synergistically triggered insulin and GLP-1 secretion. Gs-coupled receptor stimulation primarily triggered GLP-1 secretion, which was amplified by high glucose levels in GLUTag cells. Pasireotide drastically inhibited GLP-1 secretion induced by Gs-coupled receptor stimulation through SSTR5-Gi-dependent inhibition of cAMP levels, suggesting that the main pathway was completely blocked. Furthermore, administering GLP-1 partially overcame the inhibitory effect of pasireotide in GLP-1R-MIN-6 cells, leading to a partial recovery of insulin secretion. The drastic inhibition of GLP-1 secretion via shutdown of the main pathway is the primary cause of pasireotide-induced hyperglycemia. GLP-1 analogs, rather than DPP-4 inhibitors, can spare pasireotide-induced depletion of endogenous GLP-1 and restore insulin secretion.
Keywords: GLP-1; GLP-1 analog; GPCRs; Insulin; L-cells; Pasireotide.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

