ERG1A K+ channel increases intracellular calcium concentration through modulation of calsequestrin1 in C2C12 myotubes
- PMID: 40108273
- PMCID: PMC11923081
- DOI: 10.1038/s41598-025-93788-7
ERG1A K+ channel increases intracellular calcium concentration through modulation of calsequestrin1 in C2C12 myotubes
Abstract
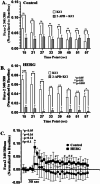
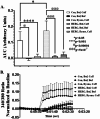
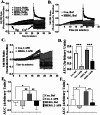
The ERG1A K+ channel modulates the protein degradation that contributes to skeletal muscle atrophy by increasing intracellular calcium concentration ([Ca2+]i) and enhancing calpain activity, but the mechanism by which the channel regulates the [Ca2+]i is not known. Here, we have investigated the effect of human ERG1A (HERG) on [Ca2+]i in C2C12 myotubes, using Fura-2 calcium assays, immunoblot, RT-qPCR, and electrophysiology. The data show that the rise in [Ca2+]i induced by KCl-stimulated depolarization is of greater amplitude in C2C12 myotubes over-expressing HERG relative to controls, but this difference does not result from an increase in L-type channel (Cav1.1) Ca2+ influx because there is no statistical difference in the nifedipine-sensitive response upon depolarization between the expression groups. Indeed, HERG overexpression in C2C12 myotubes has no effect on the amplitude of L-type channel current nor does it affect the mRNA levels nor protein abundance of the Cav1.1 channel. This finding suggests that HERG modulates excitation coupled calcium entry (ECCE). Indeed, the HERG-enhanced increase in [Ca2+]i induced by depolarization is blocked by 2-aminoethoxydiphenyl borate, an inhibitor of ECCE. Further, HERG also modulates the activity of ryanodine receptors (RYR1, a component of ECCE) as well as store operated calcium entry (SOCE). Therefore, we investigated the effect of HERG on calsequestrin1, a calcium buffering/binding protein known to modulate RYR1 and SOCE activities. Indeed, we find that calsequestrin1 mRNA levels are decreased 0.83-fold (p < 0.05) and the total protein abundance is lowered 77% (p < 0.05) in myotubes over-expressing HERG relative to controls. In conclusion, the data show that ERG1A overexpression modulates [Ca2+]i in skeletal muscle cells by lowering the abundance of the calcium buffering/binding protein calsequestrin1 which interacts with RyR1 and SOCE pathways. Indeed, we report that overexpression of HERG in myotubes increases [Ca2+]i by modulation of RyR1 as well as ECCE and SOCE activities. It is likely that HERG enhancement of RyR1 activity, through decreased Casq1 abundance, is increasing [Ca2+]i. This study provides a potential mechanism to explain how upregulation of ERG1A contributes to increased [Ca2+]i and, thus, atrophy in skeletal muscle.
Keywords: Ether-a-gogo related K+ channel; Intracellular calcium; RyR1; Skeletal muscle; Store operated calcium entry; calsequestrin1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures






References
-
- London, B. et al. Two isoforms of the mouse Ether-a-go-go-related gene coassemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ. Res.81, 870–878 (1997). - PubMed
-
- Lees-Miller, J. P., Kondo, C., Wang, L. & Duff, H. J. Electrophysiological characterization of an alternatively processed ERG K+ channel in mouse and human hearts. Circ. Res.81(5), 719–726 (1997). - PubMed
-
- Curran, M. E. et al. A molecular basis for cardiac arrhythmia: Herg mutations cause long QT syndrome. Cell80, 795–803 (1995). - PubMed
-
- Jones, E. M. C., Roti Roti, E. C., Wang, J., Delfosse, S. A. & Robertson, G. A. Cardiac IKr channels minimally comprise hERG1a and 1b subunits. J. Biol. Chem.279, 44690–44694 (2004). - PubMed
-
- Wang, X. et al. Merg1a K+ channel induces skeletal muscle atrophy by activating the ubiquitin proteasome pathway. FASEB J.20(9), 1531–1533 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

