Credibility assessment of a mechanistic model of atherosclerosis to predict cardiovascular outcomes under lipid-lowering therapy
- PMID: 40108310
- PMCID: PMC11923190
- DOI: 10.1038/s41746-025-01557-7
Credibility assessment of a mechanistic model of atherosclerosis to predict cardiovascular outcomes under lipid-lowering therapy
Abstract
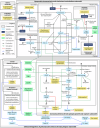
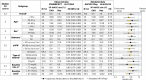
Demonstrating cardiovascular (CV) benefits with lipid-lowering therapy (LLT) requires long-term randomized clinical trials (RCTs) with thousands of patients. Innovative approaches such as in silico trials applying a disease computational model to virtual patients receiving multiple treatments offer a complementary approach to rapidly generate comparative effectiveness data. A mechanistic computational model of atherosclerotic cardiovascular disease (ASCVD) was built from knowledge, describing lipoprotein homeostasis, LLT effects, and the progression of atherosclerotic plaques leading to myocardial infarction, ischemic stroke, major acute limb event and CV death. The ASCVD model was successfully calibrated and validated, and reproduced LLT effects observed in selected RCTs (ORION-10 and FOURIER for calibration; ORION-11, ODYSSEY-OUTCOMES and FOURIER-OLE for validation) on lipoproteins and ASCVD event incidence at both population and subgroup levels. This enables the future use of the model to conduct the SIRIUS programme, which intends to predict CV event reduction with inclisiran, an siRNA targeting hepatic PCSK9 mRNA.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.A. reports having received payment or honoraria for lectures, presentations, speakers bureaus or educational events from Amgen, Alnylam, Amarin, Astra Zeneca, Boehringer, BMS, Bouchara Recordati, Pfizer, Novartis, Novo Nordisk, Organon, Sanofi, Servier, Vifor. P.A. reports: Grants: French government (PHRC: RIISC-THETIS, TST-40, SPICAF trials), Pfizer and AstraZeneca (TST trial); Speaker fee: Novartis, Viatris, Sanofi; Advisory board: Novartis, Neuraltide; Steering committee: Bayer. F.B. reports having received payments for consulting, speaking, or educational events from Amgen, Amarin, Novartis, Novo Nordisk, Boehringer, Servier, ViiV healthcare, Gilead and Sanofi. B.C. reports having received payments for consulting, speaking, or educational events from Amgen, Astra Zeneca, BMS, Eli Lilly, Novartis, Novo Nordisk, Sanofi, Ultragenyx. G.M. reports having received payments for consulting, speaking, or educational events from Amgen, Amarin, Bayer Healthcare, BMS, Leo Pharma, Novartis, Novo Nordisk, Pfizer and Sanofi. P.G.S. reports: Research grants: Amarin, Sanofi; Clinical Trials (Steering committee, CEC, DSMB): Amarin, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Idorsia, Janssen, Novartis, Novo-Nordisk, Pfizer, Sanofi; Consulting or speaking: Amarin, Amgen, BMS, Novo-Nordisk; Senior Associate Editor at Circulation; Chief Scientific Officer: Bioquantis.
Figures




References
-
- Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Prim.5, 56 (2019). - PubMed
-
- WHO. Cardiovascular diseases (CVDs). (2021) https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases....
LinkOut - more resources
Full Text Sources
Miscellaneous

