Three-year analysis of adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3
- PMID: 40108332
- PMCID: PMC12055586
- DOI: 10.1038/s41375-025-02532-7
Three-year analysis of adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3
Abstract
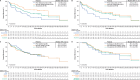
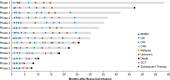
Brexucabtagene autoleucel (brexu-cel) is an autologous anti-CD19 CAR T-cell therapy approved in the US to treat adults aged ≥18 years (≥26 years in the EU) with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL). Brexu-cel showed an overall complete remission (CR)/CR with incomplete hematologic recovery (CRi) rate of 73% (CR rate 60%) and median overall survival (OS) of 25.4 months in 78 patients with R/R B-ALL after 2 years in ZUMA-3. Here, we report updated outcomes after >3 years median follow-up. As of July 23, 2022, median follow-up in all patients (N = 78) was 41.6 months. Median OS (95% CI) was 25.6 months (1.2-47.0; N = 78) and was 38.9 months (25.4-not estimable) for responders (n = 58), with 9 patients in ongoing remission without subsequent therapies. Five deaths (none deemed brexu-cel-related) occurred since prior data cut. Benefits from brexu-cel were maintained regardless of age, prior therapies, and subsequent allogeneic stem cell transplantation (alloSCT). Subsequent alloSCT was not associated with survival benefit among responders versus responders without subsequent alloSCT. No secondary T-cell malignancies were reported in ZUMA-3 with long-term follow-up.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: BDS reports consulting/advisory role for Adaptive Biotechnologies, Amgen, Autolus, BeiGene, Bristol Myers Squibb, Century Therapeutics, Deciphera, Jazz, Kite, a Gilead Company, Lilly, Novartis, Precision Biosciences, Pfizer, and Takeda; research funding from Jazz Pharmaceuticals, Kite, and Servier; and other relationship with PeproMene Bio. RDC reports employment with and stock or other ownership in Seagen (immediate family member); honoraria from Amgen, Jazz, Kite, a Gilead Company, and Pfizer; consultancy/advisory role for Amgen and Kite; research funding from Amgen, Incyte, Kite, Merck, Pfizer, Servier, and Vanda; travel support from Kite; and other relationship with Autolus (independent review committee), and PeproMene Bio (DSMB). JHP reports consulting/advisory role for AstraZeneca, Kite, a Gilead Company, and Novartis; and research funding from Amgen, Genentech, and Juno. RH reports honoraria from ADC Therapeutics, Bristol Myers Squibb, Celgene, Gilead Sciences, Janssen, Kite, a Gilead Company, MSD, and Novartis; and consulting/advisory role for Kite and Gilead Sciences. ACL reports consulting/advisory role for AbbVie, Actinium, Bristol Myers Squibb, Pfizer, and Takeda; research funding from Amgen, Astellas, Autolus, Kadmon, Kite, a Gilead Company, Pharmacyclics, and Talaris; and other relationship with DSMB: Servier. NB reports honoraria and consulting/advisory role for Amgen, Kite, a Gilead Company, and Novartis; and research funding and expert testimony for Amgen. TL reports honoraria from Amgen, Incyte, Kite, a Gilead Company, and Servier; and consulting/advisory role for Amgen, Incyte, and Servier. MiRB reports honoraria from ADC Therapeutics, Bristol Myers Squibb, Incyte, and Sanofi; consulting/advisory role for Bristol Myers Squibb, Kite, a Gilead Company, Novartis, and Sana Bio; and speakers’ bureau participation for ADC Therapeutics, Bristol Myers Squibb, Incyte, and Sanofi. MST reports consulting/advisory role for AstraZeneca, Bristol Myers Squibb, Genmab, Kite, a Gilead Company, and Roche; research funding from Kite, Regeneron, Roche, and Takeda; and travel support from Janssen and Kite. KMO has no relevant financial relationships to disclose. DT reports consulting/advisory role for and research funding from Bristol Myers Squibb. MLA reports consulting/advisory role for Kite, a Gilead Company and Syndax Pharmaceuticals, Inc.; and research funding from Kite (Institutional PI). YL reports consulting/advisory role for Janssen, Sanofi, NexImmune, Caribou, BMS, Pfizer, Regeneron; research funding from BMS and Jansen. MRB reports research funding from AbbVie, Ascentage, Kite, a Gilead Company, Kura, and Takeda. GJS reports honoraria and research funding from and speakers’ bureau participation for Kite, a Gilead Company. MS reports consulting/advisory role for Amgen, Celgene/Bristol Myers Squibb, Gilead Sciences, Janssen, and Novartis; speakers’ bureau participation for Celgene/Bristol Myers Squibb, Gilead Sciences, Janssen, and Novartis; research funding from Amgen, Gilead Sciences, Miltenyi Biotec, MorphoSys, Roche, and Seagen; and travel support from Takeda. MA reports honoraria from and consulting/advisory role for Celgene; speakers’ bureau participation for AbbVie, Bristol Myers Squibb, Celgene, and Kite, a Gilead Company. MCM reports consulting or advisory role for Bristol Myers Squibb, CDR-life, GSK, and Janssen/Cilag (institution); speakers’ bureau participation for WebMD, Siemens, and Pfizer (institution); and research funding from BeiGene (institution). WGW reports consulting/advisory role for Genzyme and Sanofi; and research funding from AbbVie, Acerta, Cyclacel, Genentech, Gilead Sciences, GSK, Janssen, Juno, Karyopharm, Loxo Oncology, miRagen, Novartis, Oncternal, Pharmacyclics, Sunesis, and Xencor. DJD reports consulting or advisory role for Agios, Amgen, Autolus, Blueprint, Forty-Seven, Gilead Sciences, Incyte, Jazz, Novartis, Pfizer, Shire, and Takeda; and research funding from AbbVie, GlycoMimetics, Novartis, and Blueprint Pharmaceuticals. PS reports honoraria from and consulting or advisory role for MorphoSys and CRISPR Therapeutics; and research funding from Amgen, Gamida Cell, Pfizer, Karyopharm, Gilead Sciences, Incyte, Seagen, and Cellectar. DJ reports research funding from Jazz Pharmaceuticals and Pfizer. DM has no relevant financial relationships to disclose. SA reports employment with and stock or other ownership in Kite, a Gilead Company. LZ reports employment with Kite, a Gilead Company; and stock or other ownership in AbbVie RSU. TH reports employment with, stock, or other ownership in Kite, a Gilead Company. RDK reports employment with, stock or other ownership in, and travel support from Kite, a Gilead Company. AG reports honoraria from Kite, a Gilead Company; consulting/advisory role for Amgen, Atara, Bristol Myers Squibb, CRISPR Therapeutics, Kite, and Wugen Inc.; research funding from Amgen, Genentech, and Kite. OOO reports honoraria from Gilead Sciences and Pfizer; consulting/advisory role for AbbVie, ADC, Cargo, Caribou Biosciences, Epizyme, Gilead Sciences, Kite, a Gilead Company, Nektar, Novartis, Pfizer, and TGR; speakers’ bureau participation for ADC; and research funding from Allogene, Daiichi Sankyo, Kite, and Pfizer.
Figures


References
-
- Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gokbuget N, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125:2474–87. - PMC - PubMed
-
- Aldoss I, Yang D, Malki MMA, Mei M, Mokhtari S, Artz A, et al. Allogeneic Hematopoietic Cell Transplantation for Relapsed and Refractory Philadelphia Negative B Cell ALL in the Era of Novel Salvage Therapies. Transpl Cell Ther. 2021;27:255.e1–e9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

