Sleep pressure accumulates in a voltage-gated lipid peroxidation memory
- PMID: 40108451
- PMCID: PMC12043502
- DOI: 10.1038/s41586-025-08734-4
Sleep pressure accumulates in a voltage-gated lipid peroxidation memory
Abstract
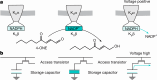
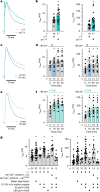
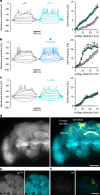
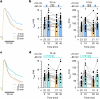
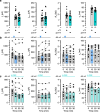
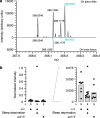
Voltage-gated potassium (KV) channels contain cytoplasmically exposed β-subunits1-5 whose aldo-keto reductase activity6-8 is required for the homeostatic regulation of sleep9. Here we show that Hyperkinetic, the β-subunit of the KV1 channel Shaker in Drosophila7, forms a dynamic lipid peroxidation memory. Information is stored in the oxidation state of Hyperkinetic's nicotinamide adenine dinucleotide phosphate (NADPH) cofactor, which changes when lipid-derived carbonyls10-13, such as 4-oxo-2-nonenal or an endogenous analogue generated by illuminating a membrane-bound photosensitizer9,14, abstract an electron pair. NADP+ remains locked in the active site of KVβ until membrane depolarization permits its release and replacement with NADPH. Sleep-inducing neurons15-17 use this voltage-gated oxidoreductase cycle to encode their recent lipid peroxidation history in the collective binary states of their KVβ subunits; this biochemical memory influences-and is erased by-spike discharges driving sleep. The presence of a lipid peroxidation sensor at the core of homeostatic sleep control16,17 suggests that sleep protects neuronal membranes against oxidative damage. Indeed, brain phospholipids are depleted of vulnerable polyunsaturated fatty acyl chains after enforced waking, and slowing the removal of their carbonylic breakdown products increases the demand for sleep.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.A.M. and S.G. are employees of and B.S. is a consultant for TransMIT GmbH. The other authors declare no competing interests.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

