Dynamic cytoskeletal regulation of cell shape supports resilience of lymphatic endothelium
- PMID: 40108458
- PMCID: PMC12058511
- DOI: 10.1038/s41586-025-08724-6
Dynamic cytoskeletal regulation of cell shape supports resilience of lymphatic endothelium
Abstract
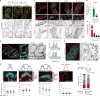
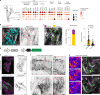
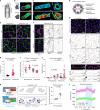
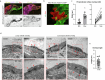
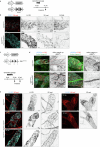
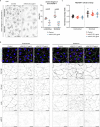
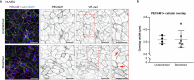
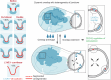
Lymphatic capillaries continuously take up interstitial fluid and adapt to resulting changes in vessel calibre1-3. The mechanisms by which the permeable monolayer of loosely connected lymphatic endothelial cells (LECs)4 maintains mechanical stability remain elusive. Here we identify dynamic cytoskeletal regulation of LEC shape, induced by isotropic stretch, as crucial for the integrity and function of dermal lymphatic capillaries. We found that the oak leaf-shaped LECs showed a spectrum of VE-cadherin-based junctional configurations at the lobular intercellular interface and a unique cytoskeletal organization, with microtubules at concave regions and F-actin at convex lobes. Multispectral and longitudinal intravital imaging of capillary LEC shape and actin revealed dynamic remodelling of cellular overlaps in vivo during homeostasis and in response to interstitial fluid volume increase. Akin to puzzle cells of the plant epidermis5,6, LEC shape was controlled by Rho GTPase CDC42-regulated cytoskeletal dynamics, enhancing monolayer stability. Moreover, cyclic isotropic stretch increased cellular overlaps and junction curvature in primary LECs. Our findings indicate that capillary LEC shape results from continuous remodelling of cellular overlaps that maintain vessel integrity while preserving permeable cell-cell contacts compatible with vessel expansion and fluid uptake. We propose a bellows-like fluid propulsion mechanism, in which fluid-induced lumen expansion and shrinkage of LEC overlaps are countered by actin-based lamellipodia-like overlap extension to aid vessel constriction.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: F.L., S. Schurmann and O.F. have filed a patent application related to the MultiStretcher device as inventors (DPMA 10 2023 205 399.3). The other authors declare no competing interests.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

