Structural dynamics of DNA unwinding by a replicative helicase
- PMID: 40108462
- PMCID: PMC12043514
- DOI: 10.1038/s41586-025-08766-w
Structural dynamics of DNA unwinding by a replicative helicase
Abstract
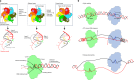
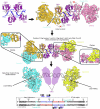
Hexameric helicases are nucleotide-driven molecular machines that unwind DNA to initiate replication across all domains of life. Despite decades of intensive study, several critical aspects of their function remain unresolved1: the site and mechanism of DNA strand separation, the mechanics of unwinding propagation, and the dynamic relationship between nucleotide hydrolysis and DNA movement. Here, using cryo-electron microscopy (cryo-EM), we show that the simian virus 40 large tumour antigen (LTag) helicase assembles in the form of head-to-head hexamers at replication origins, melting DNA at two symmetrically positioned sites to establish bidirectional replication forks. Through continuous heterogeneity analysis2, we characterize the conformational landscape of LTag on forked DNA under catalytic conditions, demonstrating coordinated motions that drive DNA translocation and unwinding. We show that the helicase pulls the tracking strand through DNA-binding loops lining the central channel, while directing the non-tracking strand out of the rear, in a cyclic process. ATP hydrolysis functions as an 'entropy switch', removing blocks to translocation rather than directly powering DNA movement. Our structures show the allosteric couplings between nucleotide turnover and subunit motions that enable DNA unwinding while maintaining dedicated exit paths for the separated strands. These findings provide a comprehensive model for replication fork establishment and progression that extends from viral to eukaryotic systems. More broadly, they introduce fundamental principles of the mechanism by which ATP-dependent enzymes achieve efficient mechanical work through entropy-driven allostery.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











Similar articles
-
Mechanisms of conformational change for a replicative hexameric helicase of SV40 large tumor antigen.Cell. 2004 Oct 1;119(1):47-60. doi: 10.1016/j.cell.2004.09.017. Cell. 2004. PMID: 15454080
-
Molecular Basis for ATP-Hydrolysis-Driven DNA Translocation by the CMG Helicase of the Eukaryotic Replisome.Cell Rep. 2019 Sep 3;28(10):2673-2688.e8. doi: 10.1016/j.celrep.2019.07.104. Cell Rep. 2019. PMID: 31484077 Free PMC article.
-
Study of SV40 large T antigen nucleotide specificity for DNA unwinding.Virol J. 2017 Apr 14;14(1):79. doi: 10.1186/s12985-017-0733-5. Virol J. 2017. PMID: 28410592 Free PMC article.
-
Grip it and rip it: structural mechanisms of DNA helicase substrate binding and unwinding.Protein Sci. 2014 Nov;23(11):1498-507. doi: 10.1002/pro.2533. Epub 2014 Aug 22. Protein Sci. 2014. PMID: 25131811 Free PMC article. Review.
-
Mechanisms of replication origin licensing: a structural perspective.Curr Opin Struct Biol. 2019 Dec;59:195-204. doi: 10.1016/j.sbi.2019.08.007. Epub 2019 Oct 17. Curr Opin Struct Biol. 2019. PMID: 31630057 Review.
Cited by
-
Structure of the human CTF18-RFC clamp loader bound to PCNA.bioRxiv [Preprint]. 2025 Jul 24:2024.05.08.593111. doi: 10.1101/2024.05.08.593111. bioRxiv. 2025. PMID: 40777363 Free PMC article. Preprint.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

