Matrix-producing neutrophils populate and shield the skin
- PMID: 40108463
- PMCID: PMC12074881
- DOI: 10.1038/s41586-025-08741-5
Matrix-producing neutrophils populate and shield the skin
Erratum in
-
Author Correction: Matrix-producing neutrophils populate and shield the skin.Nature. 2025 May;641(8065):E10. doi: 10.1038/s41586-025-09082-z. Nature. 2025. PMID: 40335705 No abstract available.
Abstract
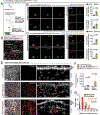
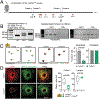
Defence from environmental threats is provided by physical barriers that confer mechanical protection and prevent the entry of microorganisms1. If microorganisms overcome those barriers, however, innate immune cells use toxic chemicals to kill the invading cells2,3. Here we examine immune diversity across tissues and identify a population of neutrophils in the skin that expresses a broad repertoire of proteins and enzymes needed to build the extracellular matrix. In the naive skin, these matrix-producing neutrophils contribute to the composition and structure of the extracellular matrix, reinforce its mechanical properties and promote barrier function. After injury, these neutrophils build 'rings' of matrix around wounds, which shield against foreign molecules and bacteria. This structural program relies on TGFβ signalling; disabling the TGFβ receptor in neutrophils impaired ring formation around wounds and facilitated bacterial invasion. We infer that the innate immune system has evolved diverse strategies for defence, including one that physically shields the host from the outside world.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

